
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
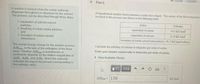
Transcribed Image Text:Part C
A solution is formed when the solute unilormly
disperses throughout (or dissolves in) the solvent
The process can be described though three stops
A hypothetical solution forms between a solid and a liquid The vakues of the thermodynamic
Involved in the process are shown in the following table
1 separation of solvent-solvant
particles,
2. breaking of solute-solute particles
and
3. formation of solute-solvent
interactions
Action
Enthalpy
145 kJ/mol
21.8 kJ/mol
séparation of solute
separation of solvent
formation of solute-solvent interactions -85.7 kJ/mol solute
The overall energy change for the solution process,
AHa is the sum of the enthalpies of the threoe
stepo. Whether AHs endothermic or
exothermic depends on the relative magnitudes of
AH, AH, and AH, where the subscript
Indicates the step in the process corresponding to
the enthalpy value
Calculate the enthalpy of solution in kilojoules per mole of solute
Enter your answer numerically in kilojoules per mole of solute.
> View Available Hint(s)
V AE
AH= 136
El/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A solution of a nonelectrolyte solution contains 39.0 g of solute dissolved in 242.0 g of water. The freezing point of the water is observed to be -4.00˚C. The Kf for water is 1.86 ˚C/m and normal freezing point of 0.00˚C. What is the molar mass of the substance? -2.50˚C. The Kf for water is 1.86 ˚C/m and normal freezing point of 0.00˚C. What is the molar mass of the substance?arrow_forwardA student mistakenly uses 0.31g Mg. Will the temperature change of the solution be affected/ If so, will it be larger or smaller that it would be have been using 0.15g mg? Explain. The amount of heat released is (independent of the quality, a molar quality, depends on the temperature)arrow_forwardWhen working with molality, and no amount of solvent is specified, it may be convenient to assume Molality (m): Moles of solute per kilograms of solvent. Molality moles of solute kilograms of solvent You have 1 kg of solution. You have 1 kg of solvent. You have 1 L of solvent. You can never make assumptions about molality.arrow_forward
- A hypothetical solution forms between a solid and a liquid. The values of the thermodynamic quantities involved in the process are shown in the following table. Action Enthalpy separation of solute 12.5 kJ/mol separation of solvent 28.8 kJ/mol formation of solute-solvent interactions -88.7 kJ/mol solute Calculate the enthalpy of solution in kilojoules per mole of solute. Enter your answer numerically in kilojoules per mole of solute.arrow_forwardThe freezing point of a CaCl₂ solution formed by dissolving 0.020 mol of CaCl₂ in 1,090 g of H₂O is-0.0397 °C. CaCl₂ dissociates as follows: Data: Ky 1.86 kg.K/mol The % dissociation, rounded to two significant figures, of CaCl₂ is: Number CaCl₂ Ca²+2 Ch %arrow_forwardDetermine the osmotic pressure of a 119.1 L aqueous solution that contains 3.1 g of NaI at 23 C. The molar mass of NaI is 149.89 g/mol. R = 8.314 J/mol*K or 0.0821 L*atm/(mol*K) Round your answer to contain two significant figures. Your Answer:arrow_forward
- Which of the following three solute/solvent pairs are insoluble? Choose one or more: benzene/toluene Benzene Toluene acetone/ammonia Acetone H. N. H. Ammonia mercury/water Hg Mercury O. H. Water O butane/ethanol Butane H. Ethanol water/xylene H' H. Water Xylenes propane/octane Propane Octanearrow_forwardMoles of solute (mol)Use the molality to convert kg of solvent to moles of solute. Hints: molality of the solution (m) 0.76612903225806 Mass of solvent in grams 151.5 Mass of solvent in kilograms 0.1515arrow_forward(Q73) A solution contains 16.90 grams of magnesium chloride and 815.7 grams of water. What is the vapor pressure (in torr) of this solution at 40.00°C? The vapor pressure of water at this temperature is 55.30 torr. You should use the experimental value of the van't Hoff factor for this calculation. (4 sf)arrow_forward
- Given: Mass of lauric acid (g): 8.000g Mass of benzoic acid (g): 1.027g Freezing temperature of pure lactic acid(°C): 43.3°C Freezing point of the benzoic acid-lauric acid mixture(°C): 39.8°C m of lauric acid: 1.8m Mols of benzoic acid solute: .0072mol Question: Calculate the experimental molecular weight of benzoic acid, in g/mol.arrow_forwardWhat is the boiling point of a solution that contains 1.00 kg of water and each of the following quantities of solute?a. 2.00 moles of sucrose (a molecular compound) b. 2.00 moles of fructose (a molecular compound) c. 2.00 moles of Na2SO4 (an ionic compound) d. 2.00 moles of KCl (an ionic compound)arrow_forwardCalculate the freezing point of a solution containing 74.0 g of eucalyptol (C10H8O) solute dissolved in 0.600 kg of CHCl3. The freezing point of pure CHCl3 is -63.5 oC. CHCl3 has a Kf of 4.68 oC/molality. -64.9 oC -67.5 oC -48.3 oC -58.0 oC -69.0 oC -62.1 oC -59.5 oC -78.7 oCarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY