
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
After adding 6M HCl to the unknown solution, the resulting solid is separated from the liquid. Hot water is next added to the solid. Not mixing the solid real well with the hot water could result in false-positive results for which ion(s)?
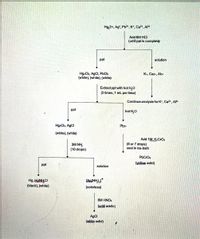
Transcribed Image Text:Hg,2+, Ag*, Pb2*, K*, Ca2", AP+
Add 6M HCI
(until ppt is complete)
ppt
solution
Hg2Cl2, AgCI, PbCl2
(white), (white), (white)
K+, Ca2+, Al3+
Extract ppt with hot H,O
(3 times, 1 mL per time)
Continue analysis for Kt, Ca2+, AP+
ppt
hot H,O
Hg2Cl2, AgCI
Pb2+
(white), (white)
Add 1M K2CrO4
3M NH
(10 drops)
(6 or 7 drops)
cool in ice-bath
PbCrO4
(yellow solid)
ppt
solution
+
[Ag{NH),j*
(black), (white)
(colorless)
6M HNO3
(until acidic)
AgCl
(white solid)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ⚫ To 3 mL of 0.10 M HC_H₂O2 add a drop of methyl orange, then add 1 M NaC HO₂ a few 3 2 3 drops at a time, with mixing. Explain your observations in terms of the equilibrium equation and the common ion effect. • Write the equilibrium equation and explain your observations in terms of the equilibrium equation and the common ion effect in your notebook.arrow_forwardPlease help solve this problemarrow_forwardQuestion 9 of 9 Šubmit Determine the pH at the point in the titration of 40.0 mL of 0.200 M H,NNH, with 0.100 M HNO, after 10.0 mL of the strong acid has been added. The value of Kb for H,NNH, is 3.0 x 10°. 1 2 NEXT> Use the table below to determine the moles of reactant and product after the reaction of the acid and base. H,NNH,(aq) + H*(aq) H,NNH,"(aq) Before (mol) Change (mol) After (mol) CRESET 0.200 0.100 1.00 x 103 -1.00 x 103 2.00 x 103 -2.00 x 10* 6.00 x 103 -6.00 x 103 7.00 x 103 -7.00 x 103 8.00 x 103 -8.00 x 103arrow_forward
- arrow_forward1. The acid form of bromophenol blue (???) is yellow; the base form (??−) is blue. Approximately what wavelength of visible light do you think each of these forms will absorb? Briefly explain. 2. Solution ?3 is considered to be the acid form of the indicator. Solution ?3 is considered to be the basic form of the indicator. Consider Le Chatelier’s Principle and explain why this is so.arrow_forwardAbove what Fe2+ concentration will Fe(OH, precipitate from a buffer solution that has a pH of 9.72 ? The Rsp Of Fe(OH), is 4.87 \times 10 - 17:arrow_forward
- Buffers will resist pH changes following the addition of acid or base within about +/-1 pH unit of the pka of a solution. True Falsearrow_forwardCalculate the percent ionization of a 0.15 M benzoic acid solution in pure water and in a solution containing 0.10 M sodium benzoate. Why does the percent ionization differ significantly in the two solutions?arrow_forwardDetermine the resulting pH when 0.003 mol of solid NaOH is added to a 100.0 mL buffer containing 0.13 M HClO and 0.37 M NaClO. The value of Ka for HClO is 2.9 × 10⁻⁸. Determine the moles of the ractant and product after the reaction of the acid and base. (Similar to ICE format but is instead Before (mol), Change (mol), and After (mol) Determine the ICE table for HClO (aq) + H2O - H3O+ + ClO- (aq) Fill in Ka= ? = 2.9 * 10-8 Calculate pHarrow_forward
- 4) calculate the pH of the buffer solution after the addition of 0.15 mL of sodium hydroxide based on the known value of Ka for acetic acid? 5) calculate the pH of water after the addition of 0.15 mL of 1 M NaOH? *buffer solution by mixing 20.00 mL of 0.100 M sodium hydroxide and 40.00 mL of 0.100 M acetic acidarrow_forwardBromthymol blue has a Ka value of 1.0 \times 10-7, is yellow in its HIn form and blue in its In form. Suppose we put a few drops of this indicator in a strongly acidic solution. If the solution is then titrated with NaOH, at what pH will the indicator color change first be visible?arrow_forwardCalculate the expected pH based on how the solution was prepared, again allowing for any dilution taking place. For Solutions 7 and 8, make sure to take into account the acid–base reaction that occurs when an acid or base is added to the buffer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY