
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
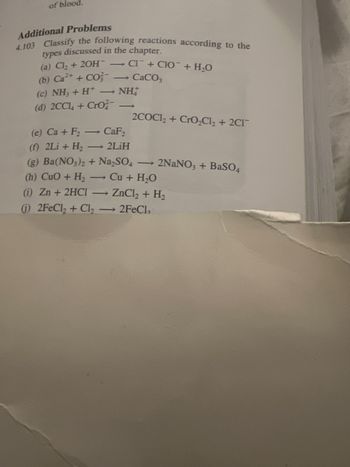
Transcribed Image Text:of blood.
Additional Problems
4.103 Classify the following reactions according to the
types discussed in the chapter.
(a) Cl₂ + 2OH-CI+CIO + H₂O
(b) Ca²+ + CO - CaCO,
(c) NH3 + H* — NH
(d) 2CCl4 +Cro
2COC1₂+ CrO₂Cl₂ + 2C1
(e) Ca + F₂ - CaF₂
(f) 2Li + H₂ - 2LiH
(g) Ba(NO3)2 + Na₂SO4 → 2NaNO3 + BaSO4
(h) CuO + H₂ →→ Cu + H₂O
(i) Zn + 2HCl → ZnCl₂ + H₂
(j) 2FeCl₂ + Cl₂ → 2FeCl₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the following chemical equations.arrow_forwardDecomposition Combination Single Replacement Double Replacement Are my optionsarrow_forwardWhen each of the following pairs of aqueous solutions is mixed, does a precipitation reaction occur? If so, write the formula and name of the precipitate. (Type your answer using the format CO2 for CO2. Type NONE in the blanks if there is no precipitation reaction.) (a) lead(II) nitrate + potassium bromideformulaname(b) sodium sulfide + nickel(II) sulfateformulanamearrow_forward
- What type (redox, acid-base, precipitation) are the reactions below? For the redox reactions, identify the element that is oxidized and reduced, as well as the oxidizing and reducing agent. For the elements that are oxidized or reduced indicate the oxidation number before and after the reaction occurs. (a) FeCl3 + Cu ⟶ FeCl2 + CuCl (b) CuSO4 + Pb(NO3)2 ⟶ Cu(NO3)2 + PbSO4 (c) NH4NO3 ⟶ N2O + 2 H2Oarrow_forwardQ4(B) Define mass number. Explain the following redox reactions.arrow_forward4.26 Write balanced net ionic equations for the reactions that oc- cur in each of the following cases. Identify the spectator ion or ions in each reaction. (а) Cr./(SO,);(ag) + (NH,),CO,(аg) (b) Ва(NO;)2(ag) + K,SO,(aq) (c) Fe(NO3)2(aq) + KOH(aq)arrow_forward
- 5. Write the equations for the neutralization reactions (a) between Magnesium Hydroxide and Sulfuric acid and (b) Barrium hydroxide and hydrochloric acid.arrow_forwardComplete and balance the following acid-base and acid-carbonate reactions. (Use the lowest possible whole number coefficients.) (a) HCl + Ba(OH)2 → (b) HCl + Al2(CO3)3 → (c) H3PO4 + LiHCO3 → (d) Al(OH)3 + H2SO4 →arrow_forwardDetermine the oxidation states of the elements in the compounds listed. None of the oxygen-containing compounds are peroxides or superoxides.(a) H2SO4(b) Ca(OH)2(c) BrOH(d) ClNO2(e) TiCl4(f) NaHarrow_forward
- typed answer neededarrow_forwardtrue or false(a) if a substance is oxidised, it is gaining electrons.(b) if an ion is oxidised, its oxidation number increases.arrow_forward3. Assume that 2.1429 g of Cr(NO3)3 is dissolved in enough water to make 50.00 mL of solution. (a) What is the molarity of Cr(NO3)3? (b) What is the molarity of the chromium(III) cation? (c) What is the molarity of the nitrate anion? The molar mass of Cr(NO3)3 is 238.011 g/mol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY