
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:V
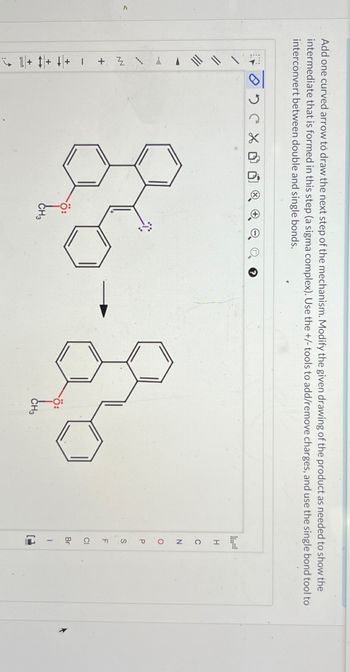
Add one curved arrow to draw the next step of the mechanism. Modify the given drawing of the product as needed to show the
intermediate that is formed in this step (a sigma complex). Use the +/- tools to add/remove charges, and use the single bond tool to
interconvert between double and single bonds.
IN Z
+
+ + +1
02CX
e o
26-å
CH3
Ö:
CH3
1
H
Z O
C
P
S
F
CI
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please don't provide handwriting solutionarrow_forwardDraw curved arrows to illustrate the mechanism for the reaction of propanol and HI.arrow_forwardAdd curved arrow(s) to draw step 2 of the mechanism. Modify the given drawing of the product as needed to show the intermediate that is formed in this step. H. C N. II P. H. Clo. CI Br HO. Ź + I +† + +1 ****arrow_forward
- Compound A is heated in methanol B to give product C. a. Draw product C and provide a detailed mechanism for its formation. OSO₂CH3 + CH3OH A A B Carrow_forwardAfter running various experiments, you determine that the mechanism for the following reaction is bimolecular. Br + OH - HO. Using this information, draw the correct mechanism in the space below. Br + Br Add/Remove step 5 � Click and drag to start drawing a structure.arrow_forwardplease help with all parts of questionarrow_forward
- How many hydrogen atoms exist on the following intermediate?arrow_forwardDraw a reaction coordinate diagram in which the structure of the transition site is more similar to the product of the reaction than to the reactant.arrow_forwardClearly label the energy diagram below when answering each part. If there is more than one specific type of label, give an appropriate number afterwards (i.e. Int. 1, Int. 2, etc). State how many step(s) are in the overall reaction, if more than one, clearly label where they start and end Identify the rate determining step (rds) Label all transition state(s) (TS) Label all intermediate(s) (Int.) Label all activation energy(ies) (Ea) Label the heat of the reaction (ΔH) State whether the overall reaction is endothermic or exothermic. please explaing each step. Thank you.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY