
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
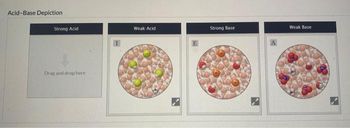
Transcribed Image Text:Acid-Base Depiction
Strong Acid
Drag and drop here
Weak Acid
E
Strong Base
Weak Base

Transcribed Image Text:Classify the molecular scenes shown according to the type of acid or base each depicts.
Scenes (3 images) (Drag and drop into the appropriate area below)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which method should be used to dilute an acidic solution to a lower molarity?arrow_forwardPlease don't provide handwritten solution ...arrow_forwardThe Bronsted-Lowry (B-L) definition of acids and bases was developed mainly because there were problems with the Arrhenius definition that kept some acids and/or bases from being classified properly. Which of the following explains the problem of the Arrhenius definition and how the Bronsted-Lowry definition help fix the classification problems. The Arrhenius definition only classified acids/bases dissolved in water as well as bases were only those that released hydroxide ions into solution. Where in the B-L definition the acid & base does not have to be in a water solution and it says a base is any substance that accepts a hydrogen ion. The Arrhenius definition only classified acids/bases dissolved in water as well as bases were only those that released hydrogen ions into solution. Where in the B-L definition the acid & base does not have to be in a water solution and it says a base is any substance that accepts a hydrogen ion. The Arrhenius definition only classified acids/bases…arrow_forward
- 3.) Which is the pH of 0.78 M HOCI (K. = 3.5 x 10*). A. 3.78 В. 3.50 С. 2.82 D. 2.53 Keywords: pH, weak acid, RICE table, conjugate base, conjugate acid, acid-ionization constant (Ka), molarity, % dissociation, Ka expression Concepts: Calculating the pH of a weak acidarrow_forward(c) NH2 Draw Your Solution HA (cat.)arrow_forward4 of 15 > Calculate the standard enthalpy of reaction, AHxn, of each of the given acid-base neutralization reactions involving the strong base KOH. The standard enthalpy of formation data are provided. $ = R Compound or Ion Standard Enthalpy of Formation (kJ/mol) -482.4 -285.8 -98.4 -349.5 -121.0 -372.1 -132.5 -80.3 -251.2 AHixn = F KOH(aq) H₂O(1) HCIO3(aq) KCIO₂ (aq) V HBr(aq) KBr(aq) NH(aq) HCIO3(aq) + KOH(aq) → KCIO3(aq) + H₂O(1) NH, (aq) K+ (aq) G Search or type URL % 5 T G B MacBook Pro 6 Y H & ve 7 N U J 00 8 M I ( 9 K O < H O L command A P Δ' 1 - ; 3 Question option [ + 11 + 21 = ? kJ/mol 11arrow_forward
- How do I solve this? I do know that it is linear.arrow_forwardIn each row check off the boxes that apply to the highlighted reactant. The highlighted reactant acts as a... (check all that apply) reaction Brønsted-Lowry acid Brønsted-Lowry base HNO,(aq) + C,H,NH,(aq) → NO,(aq) + C,H,NH (aq) olo Lewis acid Lewis base Ar Brønsted-Lowry acid Brønsted-Lowry base 2 H,0,) → 2 H,O(1) + O2(9) Lewis acid Lewis base Brønsted-Lowry acid 2+ Brønsted-Lowry base Ni2+ "(aq) + 6 NH3(aq) → Ni(NH;), (aq) 9, Lewis acid Lewis basearrow_forwardGive typed explanation of all subparts not a single word hand written otherwise leave itarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY