
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![According to the following chemical equation, how many liters at STP of hydrogen gas are needed
to make 3.00 tons of iron? (2000 lbs. = 1 ton; 454 g = 1 lb.) [Hint: The equation is not balanced.]
%D
Fe,O3 +
Н2
Fe +
Н.О
omic](https://content.bartleby.com/qna-images/question/cfef55d1-e74e-4acc-a65e-b6e34bf1c85d/dc01207d-3c72-4f8b-89cf-e0fad70f602b/8792yi9.jpeg)
Transcribed Image Text:According to the following chemical equation, how many liters at STP of hydrogen gas are needed
to make 3.00 tons of iron? (2000 lbs. = 1 ton; 454 g = 1 lb.) [Hint: The equation is not balanced.]
%D
Fe,O3 +
Н2
Fe +
Н.О
omic
Expert Solution
arrow_forward

Step 1
Amount of iron to be manufactured = 3 tonnes
The given equation for the reaction is

arrow_forward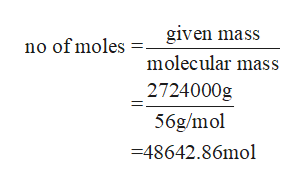

Step 2
mass of iron given = 3ton x 2000lb/ton x 454g/lb= 2724000g
No of moles of iron to be manufactured
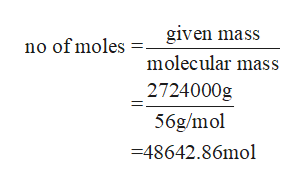
arrow_forward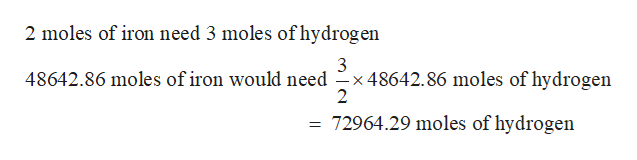

Step 3
Amount of hydrogen needed
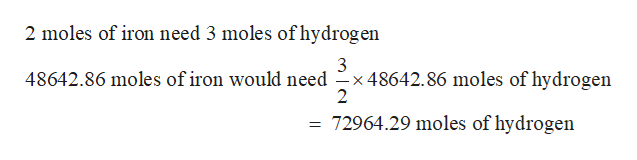
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Similar questions
- Answer the following with neat solutions. Will upvote who will answer it all and completely.arrow_forwardAssume the molecular mass of air to be 28.5 g/mol. How many moles of air are above 1 square inch of sea level (all the way to outer space)?arrow_forwardHow many liters of oxygen gas are needed to react with 1.3 x10 23 molecules of glucose?arrow_forward
- 2 H3PO4(s) --> 3 H2(g) + 2 P(s) + 4 O2(g) Using the reaction above, show step by step how many liters of oxygen can be created if I start with 0.7156 grams of H3PO4 solid at STP?arrow_forwardHow many liters of sulfur dioxide gas can be produced at STP by the addition of 9.62 mol of hydrochloric acid (HCl) to sodium sulfite if sodium chloride and water are also produced?arrow_forwardWhat volume of N2, measured at 24 °C and 719 mm Hg, will be produced by the decomposition of 14.5 g NaN3?2 NaN3(s) 2 Na(s) + 3 N2(g)arrow_forward
- How many liters of sulfur dioxide gas can be produced at STP by the addition of 9.98 mol of hydrochloric acid (HCl) to sodium sulfite if sodium chloride and water are also produced?arrow_forwardWhat amount of water (in moles) is produced by the reaction of 6.00 mol C,H12 with 36.0 mol O2? CSH12{g) + 8 O2(g)5 CO2(g) + 6 H,0(g)arrow_forwardDoes the oxygen labeled "?" come from oxygen A, oxygen B, or from water generated in the reaction? B OH OH ?arrow_forward
- What volume of O2 (MW = 32.00 g/mol) at STP is required for the complete combustion of 152.26 g CS2 (76.13 g/mol)?arrow_forwardd) Using the Ideal Gas Law, determine the experimental moles of hydrogen gas collected. e) Calculate the % yield of the reaction based on the actual yield (d) and the theoretical yield (b) calculations above.arrow_forward8. In one lab this semester, you ran the reaction: KC1O3 (s) → KCl (s) + O2 (g). The directions asked you to add about 1.5 g KC1O3 to a crucible. How many liters of O2 should form at STP?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY