
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Give detailed Solution with explanation needed with structures...draw the resonance structure. Don't give Handwritten answer
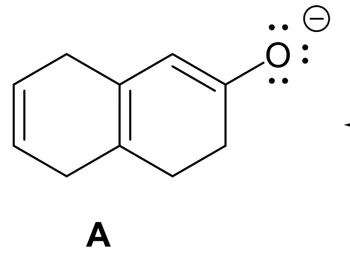
Transcribed Image Text:A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Determine the number of resonance structures frot the follwoing molecule, include the original sturcture in the tally.arrow_forwardClick the "draw structure" button to launch the drawing utility. 11 Draw a resonance structure by moving the n bonds and the radical. draw structure ...arrow_forward3) Draw all resonance structures of the following compound. Order the structures by increasing stability.arrow_forward
- A. Determine whether the curved arrow(s) shown below generate a valid or invalid resonance structure. Draw the resonance structure that would result from each properly drawn arrow and identify the arrow pushing pattern (i.e. allylic positive charge, allylic lone pair, pi bond between atoms of a different electronegativity, lone pair adjacent to a positive charge, alternating pi bonds in a ring. (a). (b).arrow_forwardGive clear detailed Solution with explanation needed of all parts..give answer all parts if you not then don't give answer..don't give Handwritten answerarrow_forward.) Draw a valid resonance structure for the following. Include the appropriate arrow to indicate your structure and the given structure are resonance structures. bearrow_forward
- Draw the best LS (with lowest formal charges) for BeAt2 on a sheet of paper (NOT TO BE TURNED IN) and answer the following:(NOTE: Use the cardinal numbers 0, 1, 2, 3, and so on for any quantity required)a) give the number of lone electron pairs on Be atom. pairsb) give the number of lone electron pairs on each At atom. pairsc) what types of bonds are attached to Be? (choose correct letter from A – D): A. two double bonds C. two single bonds B. a single & double bond D. a double & triple bond d) Fill in the table below for BeAt2. For prediction of the actual bond angle, if the angle is ideal give the numerical value; if the angle is different from ideal use > (greater than) or < (less than) with the numerical value of the ideal bond angle (for e.g., < 1800). { NOTE: i) The correct spelling of the names matters. Be sure to check your spelling.ii) For the hybridization, place the superscript numbers on the same level as the letters. For…arrow_forwardGive detailed Solution with explanation needed ...don't give Handwritten answerarrow_forwarda) Draw the complete structure of propane. (Draw all hydrogen atoms.) b) Draw the structure of propane in skeleton mode or as a bond-line structure. (Do not draw the hydrogen atoms.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY