
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
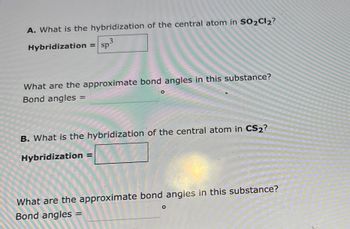
Transcribed Image Text:A. What is the hybridization of the central atom in SO2Cl2?
3
Hybridization = sp
What are the approximate bond angles in this substance?
Bond angles
=
B. What is the hybridization of the central atom in CS₂?
Hybridization
What are the approximate bond angles in this substance?
Bond angles
=
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer the following questionsarrow_forwardElement A has 6 valence electrons. Element B has 7 valence electrons. The central atom appears in one of the following periods: period 3, 4, 5, 6, or 7. They form a compound AB4. What is the molecular geometry of AB4?arrow_forwardWhat is the molecular geometry of SF6 O trigonal planar O trigonal bipyramidal Octahydralarrow_forward
- A. What is the hybridization of the central atom in BF3? Hybridization = What are the approximate bond angles in this substance? Bond angles = B. What is the hybridization of the central atom in XeO4? Hybridization = What are the approximate bond angles in this substance? Bond angles =|arrow_forwardA. What is the hybridization of the central atom in CS₂? Hybridization = What are the approximate bond angles in this substance? Bond angles = B. What is the hybridization of the central atom in BF3? Hybridization = What are the approximate bond angles in this substance? Bond angles =arrow_forwardXenon Tetrafluoride, XeF, Lewis Structure 3-D Molecular Structure Number of Valence Electrons Steric Number of Central Atom Electron Group Geometry/ Hybridization Molecular Geometry Bond Angle(s) Polar? (If yes, show dipole on 3-D structure)arrow_forward
- 16. For the molecule BrF5, what is its molecular geometry? Group of answer choices A. Square pyramid B. Octahedral C. trigonal pyramid D. tetrahedralarrow_forwardSiCl, is used as a starting material in the production of silicon polymers. Describe hybridization schemes for the central atom and the orbital overlap that occur in this halogen compound. ( Si, #Cl) 14 Data for the arrangement of electron pairs about a central atom in a molecule and geometry of the molecule and the shape of hybrid orbitals Number of Molecular Number of hybrid Hybridization of electron pairs geometry orbitals the central atom Linear 2 sp Trigonal planar 3 sp 4 Tetrahedral 4 sp 5 Trigonal 5 sp'd bipyramidal Octahedral 6. sp'd 2. 3.arrow_forwardWhat is the hybridization of the indicated atom in the following compounds (or ions)... N in N2 C in CO3 2- B in BCL3 S in SO2arrow_forward
- Which of the following factors contribute to the tetrahedral shape of the above molecule? Group of answer choices A. the shape of the two p orbitals in the carbon atom B. the shape of the one s orbital in the carbon atom C. the shape of the sp3 hybrid orbitals of the electrons shared between the carbon and hydrogen atoms D. hydrogen bonding configurations between the carbon and hydrogen atomsarrow_forwardWhat is the hybridization of the central atom in the methyl (CH3) cation? 0 X 5arrow_forwardWhat is the hybridization of the oxygen-bonded carbon atom in the following molecule? 1. S 2. p № 3. sp ற் ச் ம் 4. sp² sp³ H3C N=C=Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY