
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
. Be sure to understand what the dilution
factor is for each sample (dilution factor = the number used to determine the actual
concentration of the stock UK after the diluted samples are analyzed) ?
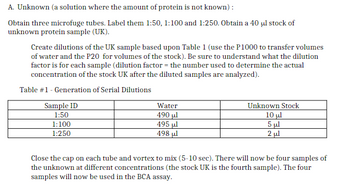
Transcribed Image Text:A. Unknown (a solution where the amount of protein is not known):
Obtain three microfuge tubes. Label them 1:50, 1:100 and 1:250. Obtain a 40 μl stock of
unknown protein sample (UK).
Create dilutions of the UK sample based upon Table 1 (use the P1000 to transfer volumes
of water and the P20 for volumes of the stock). Be sure to understand what the dilution
factor is for each sample (dilution factor = the number used to determine the actual
concentration of the stock UK after the diluted samples are analyzed).
Table #1 - Generation of Serial Dilutions
Sample ID
1:50
1:100
1:250
Water
490 ul
495 μl
498 ul
Unknown Stock
10 μl
5 μl
2 μl
Close the cap on each tube and vortex to mix (5-10 sec). There will now be four samples of
the unknown at different concentrations (the stock UK is the fourth sample). The four
samples will now be used in the BCA assay.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the % by mass of water in your hydrated copper (Il) sulphate? Mass of just anhydrous compound = mass of anhydrous compound - mass of beaker and rod 108.34g - 106.87g = 1.47g %D %3D = original compound mass - anhydrous compound mass = 3.00g - 1.47g = 1.53g Mass of just water %Darrow_forwardA student sets up the following equation to solve a problem in solution stoichiometry. (The ? stands for a number the student is going to calculate.) Enter the units of the student's answer. - 3 mol L ( 27 000) (10 - 1²+) (₁14² - - - - - - g 8.7 83.48 = L mL mol x10 X 3 μ 00 3arrow_forwardAktiv Chemistry → C ock STARTING AMOUNT + esc X https://app.101edu.co Q X N T Aktiv Chemistry 2 W S #3 X 20 F3 H E 60.5 D X + Calculate the theoretical yield in grams All, from the complete reaction of 113 grams I, according to the following balanced chemical equation: ADD FACTOR $ 4 x( ) 0.445 mol All F4 R F LL do LO % 5 113 mol I₂ F5 V 2 Al(s) + 3 (s)- -> 153.88 T Question 8 of 17 6 1 G g/mol All, 253.80 Y B 2 7 g/mol I₂ H 2 All,(s) ANSWER 407.68 F7 D 3 8¹2 N CO 8 J DII FB RESET g All 2 0.297 9 M DD K F9 121 O 7 H F10 L P F11 LL 11 F12 U ☆ delearrow_forward
- When you are presented with additional details, it can sometimes be a little more difficult to determine which information is necessary and how molarity should be applied. Using both a solution map and dimensional analysis can help clarify which information and conversion factors are necessary to determine the desired value. The following dimensional analysis setup could be used to determine the theoretical mass of AlBr3 (s) (molecular mass = 266.69 g/mol ) produced based on reacting 86.9 g of a 0.048 mol/L solution of Br2 (1) (density = 1036 g/L ) with excess Al(s) as described in the following equation: 3B12 (1) + 2A1(s) → 2AIBr3 (s) Complete the dimensional analysis for calculating the mass of the product by placing the values of each conversion factor according to whether they should appear in the numerator or denominator when calculating the mass of AlBr3 (s) produced from a sample of Br2 (1). Drag the appropriate values to their respective targets. • View Available Hint(s) Reset…arrow_forwardBy pipet, 9.00 mL of a 0.823 M stock solution of potassium permanganate (KMNO4) was transferred to a 50.00-mL volumetric flask and diluted to the calibration mark. Determine the molarity of the resulting solution. HA Value Unitsarrow_forwardBalance the chemical equationarrow_forward
- Part A 4. How many grams of silver nitrate must react to give 1.00 g of Ag? Cu(s)+2A£NO;(ag)→Cu(NO3)2 (aq)+2Ag(s) Express your answer with the appropriate units. HA μΑ MAGNO3 Value Units Submit Previous Answers Request Answer X Incorrect; Try Again Provide Feedbackarrow_forwardSolve using dimensional analysis. Show the complete solution. Do not round off nonfinal answers. Box your final answer.arrow_forward2A. Come up with a stoichiometry question with the balanced equation CH4+2O2->CO2+2H2O. This question must be solved/ answered. All work needs to be shown.arrow_forward
- Say you have a stock of 15% NaCl and need a reaction concentration of 1% in a total reaction volume of 25ul. How many ul of the 15% NaCl stock would you need to add to the reaction tube?arrow_forwardA 103.0 mL aqueous solution of 43.0 mM COCI2 is combined with 594.0 mL of aqueous 74.0 mM K3PO4. If the limiting reagent is completed consumed during this reaction, how many grams of solid precipitate will be produced? Express your answer in units of grams using at least three significant figures.arrow_forwardConsider the following chemical reaction HCl (aq) + HgNO3 (aq) à Hg2Cl2 (s) + HNO3 (aq) If 20.0 grams of HCl is mixed with 30.0 grams of HgNO3 Determine the excess reactant The amount of the solid precipitate that was recovered equals to .................. grams , if the percentage yield from this reaction was determined to be 78.4%. Write the net ionic equation for the chemical reaction Determine the type of this chemical reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY