
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
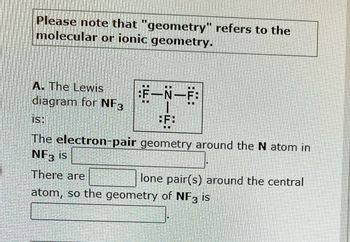
Transcribed Image Text:Please note that "geometry" refers to the
molecular or ionic geometry.
A. The Lewis
diagram for NF3
:F—N—F
BF:
On
..
is:
The electron-pair geometry around the N atom in
NF3 is
There are
atom, so the geometry of NF3 is
lone pair(s) around the central
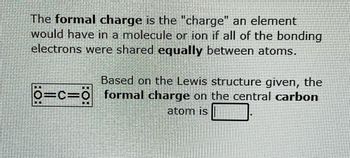
Transcribed Image Text:The formal charge is the "charge" an element
would have in a molecule or ion if all of the bonding
electrons were shared equally between atoms.
Based on the Lewis structure given, the
Ö=C=Ö formal charge on the central carbon
atom is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure :: : Cl - : 0: :0: :Z: C. C= : Z: N N Cl: Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* 0 Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". X 5arrow_forwardTo answer the questions, interpret the following Lewis diagram for NO,CI. :0-N=0 :CI: 1. For the central nitrogen atom: The number of lone pairs The number of single bonds %3D The number of double bonds 2. The central nitrogen atomarrow_forwardValence Shell Bonding Nonbonding Approx. Molecule or Lewis Electron Electron Electron VSEPR Bond Geometric Molecular Ion Structure Pairs Pairs Pairs Formula Angle Shape 1. CH4 H 4 4 AX, 109.5° tetrahedral H:C:H H 1. Complete the table (as outlined above) for the following molecules/molecular ions, all of which obey the Lewis octet rule. Complete those that are assigned by your laboratory instructor. j. SiF, k. H,S 1. NH2arrow_forward
- 0 Q: # ially Prep 3. oe aterial .Nu CIF3 Total Valence Electrons AS Chlorine trifliMoride 28 STUtUni o Lewis Dot Structure :F: :F CI:F 211 Total Electron Pairs Bonding Pairs Nonbonding Pairs Electron Geometry Molecular Geometry T-Shaped trigonal bipyramidal 4. Total Valence Electrons SF4arrow_forwardDecide whether the proposed Lewis structure below is reasonable. proposed Lewis structure Is this a reasonable structure? H. H. O Yes O Na H. O Yes O No H H H H. O Yes O No :O: O Yes H- O Noarrow_forwardThe Lewis dot structure shown contains how many single bonds- double bonds- lone pairs- s has a octet-arrow_forward
- 4. What is the total number of valence electrons in each of the following? NO2 NH3 PCI3 H2O PFs [SO.]* [PFs] +1 pon nolar.covalent bond?arrow_forwardNhy is resonance structure A and resonance structure B not the same structure? If you rotate the molecule like a spicket handle (l. e. clockwise with the C at the center and the R group remaining in place as you look down the C - R bond) wouldn't you get the same thing? Why are these not equivalent structures? Resonance structure A t Resonance structure B Actual structurearrow_forwarda. If A represents a group 5A element, what is the formal charge of A in the structure above? b. What is the shape of this molecule? c. Is this a polar molecule?arrow_forward
- The Lewis diagram for SiH4 is: I——I H-Si-H The electron-pair geometry around the Si atom in SiH4 is There is/are lone pair(s) around the central atom, so the geometry of SiH4 is The Lewis diagram for BeI₂ is: or ionic geometry. :I-Be-I: The electron-pair geometry around the Be atom in BeI₂ is There is/are lone pair(s) around the central atom, so the geometry of Bel2 isarrow_forwarda. chloroform, CHCI3 (carbon is central atom) Lewis structure Total number of electron groups around the central atom Electron geometry Molecular shape b. Carbon tetrachloride, CCI4 Lewis structure Total number of electron groups around the central atom Electron geometry Molecular shape c. Methanol, CH3OH (answer questions for the carbon center) Lewis structure Total number of electron groups around the central atom Electron geometry Molecular shapearrow_forwardDraw the Lewis Structure of BF3 Molecular Geometry? Ideal Bond Angles? Are there Polar Bonds Present (Indicate on Lewis do structure Is there an Overall Dipole? Octet rule violator and how?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY