
We discovered 50 grams of an interesting new chemical, Jerrodwowzium "JW3" on the lab bench. This chemical is not very well characterized; however, one of your fellow chemical engineering classmates was able to determine that this chemical has a molecular weight of 62. In order to clean this chemical from the lab bench 100 grams of another newly discovered chemical, Laughawazzana, LH3WH (MW 32) was added. Help me
describe this process to another engineer.
(Governing Reaction in Image)
a. Draw a block flow diagram to represent the process with only the information that has been given so far.
b. Determine which species is the limiting reactant. Do not just select one of the species.
c. For 30% conversion of the limiting reactant, show (using atomic species balances or extent of reaction) how much H2W and how much WH are produced
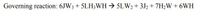
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

- Review I Constants I Periodic Table MISSED THIS? Watch KCV 7.5, WE 7.8; Read Section 7.5. You can click on the Reviow link to access the section in your e Text Part A Elemental phosphorus reacts with chlorine gas according to the equation Once the reaction has reached completion, what mass in g) of the excess reactant is let? Pa(o)+6CI, (x)-4PC(1) Express the mass in grams to three significant figures. A reaction mixture initialy contains 45.51 g Pa and 130.7g Cla. AZ4 * Submit Reauest Answer Next> Provide Feedbackarrow_forwardCalculate the Biot Number to determine if the Lumped Parameter Analysis can be used. If the Biot Number is greater than 0.10, use the Temperature-Time charts in Appendix F to calculate an answer and compare the result to the answer based on lumped capacitance. appendix F in this textbook https://drive.google.com/file/d/145Cbs8i465qUq9JhBFhia58fnPkuhU3k/view?usp=sharingarrow_forwardStart with the general mass balance relationship In – Out + Rxn = Accum and derive a formula that allows you to calculate the value of the first order reaction rate constant, k, given the half-life of a compound. Use the equation to determine the value of k for carbon tetrachloride (CCl4) given that CCl4 has a half-life of 8,030 days in air.arrow_forward
- Using the following balance equations: 2Al + 6HCl --> 2AlCl3 + 3H2 If you have 576 grams of aluminum metal and react it with 459 grams of Hydrogen Chloride, how many moles of AlCl3 can you produce?arrow_forwardA particular component of protein has a molar mass of 105 amu. It contains 34.28 % carbon, 6.726 % hydrogen, 45.67 % oxygen and 13.33 % nitrogen. Find the molecular formula of this compound. Enter your answer with the elements in the same order as they appear in the question. Also, you will not be able to make subscripts in the answer. The formula for water would be entered as H20 rather than H20. Answerarrow_forwardCarbon emissions are expected to grow exponentially from 5.5 Gt/yr to 21.4Gt/yr over the next 100 years. What would be the atmospheric concentration of CO2 in 100 years if the concentration is presently 400ppm and the mass of C in the atmosphere is presently 848 Gt?arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





