
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
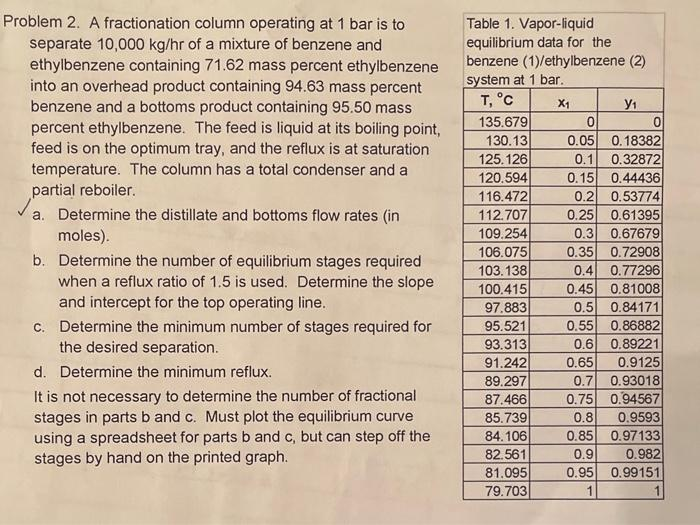
Transcribed Image Text:Problem 2. A fractionation column operating at 1 bar is to
separate 10,000 kg/hr of a mixture of benzene and
ethylbenzene containing 71.62 mass percent ethylbenzene
into an overhead product containing 94.63 mass percent
benzene and a bottoms product containing 95.50 mass
percent ethylbenzene. The feed is liquid at its boiling point,
feed is on the optimum tray, and the reflux is at saturation
temperature. The column has a total condenser and a
partial reboiler.
a. Determine the distillate and bottoms flow rates (in
moles).
b. Determine the number of equilibrium stages required
when a reflux ratio of 1.5 is used. Determine the slope
and intercept for the top operating line.
c. Determine the minimum number of stages required for
the desired separation.
d. Determine the minimum reflux.
It is not necessary to determine the number of fractional
stages in parts b and c. Must plot the equilibrium curve
using a spreadsheet for parts b and c, but can step off the
stages by hand on the printed graph.
Table 1. Vapor-liquid
equilibrium data for the
benzene (1)/ethylbenzene (2)
system at 1 bar.
T, °C
X₁
135.679
130.13
125.126
120.594
116.472
112.707
109.254
106.075
103.138
100.415
97.883
95.521
93.313
91.242
89.297
87.466
85.739
84.106
82.561
81.095
79.703
У1
0
0
0.05 0.18382
0.1
0.32872
0.15
0.2
0.25
0.3
0.35
0.4
0.45
0.5
0.9
0.95
1
0.44436
0.53774
0.61395
0.67679
0.72908
0.77296
0.81008
0.84171
0.55
0.86882
0.6 0.89221
0.65
0.9125
0.7 0.93018
0.75 0.94567
0.8
0.9593
0.85
0.97133
0.982
0.99151
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 5 images

Knowledge Booster
Similar questions
- 5. The following table shows data from a flow study of a PS melt at 210°C in a capillary rheometer. a. Make a plot of log shear stress versus log shear rate. b. Calculate the value of the viscosity at 1 and 135 s', respectively. Shear Stress ,(10° dyn/cm') Shear Rate, 3.5 1.0 12.0 5.0 20.0 12.2 33.0 25.0 40.0 52.5 60.0 135.0 69.8 400.0arrow_forwardDescribe the formation of hydrocarbon from organic matter.Explain the 'flow separation phenomena' for flow over a cylinder in cross flow. Can flow separation occur over a flat platearrow_forward
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The