
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
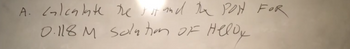
Transcribed Image Text:A. calculate the just and the POH FOR
0.118 M solution of Helly
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- O D. K = 1 OE K 1 QUESTION 25 A solution is prepared by adding 0.10 mol of iron(III) chlorite, Fe(CIO)3, to 1.00 L of water. Which statement about the solution is correct Periodic table.pdf OA. The solution is basic. O B. The solution is neutral. OC. The solution is acidic. O D. The values of Ka and Kb for the species in solution must be known before a prediction can be made. Type here to search e も DOLLarrow_forward1. Calculate the pH of a solution that is 0.0400 M in a) NaH,PO, b) NaHC2O. c) NaHSarrow_forwardAn aqueous hydrogen chloride solution reacts with water as follows HCl(aq) + H2O(L) > H3O+(aq) + Cl-(aq) this reaction is classified as a. Dissociation b. Separation c. Ionization d. None of the abovearrow_forward
- What is the pH of the solution after the reaction of HF (aq) and KOH (aq)? A. neutral B. basic C. acidicarrow_forwardA solution is a mixture of 0.100M weak acid and 0.020M of its conjugate base. The pH of the resulting solution is 5.20. What is the pKa of the weak acid? a. 7.00 b. 4.50 c. 5.90 d. 4.74 e. 8.24arrow_forwardacidic solution. SO n.arrow_forward
- Calculate the PH and POH for a .023 mole solution of HBrO2 Ki for HBrO2 = 2.1 X 10-4arrow_forward14. Calculate and select the pOH of a 0.225 M solution of N2H4 (ag) (hydrazine, a weak base) if the percent hydrolysis was found to be 0.1868% 1. 4.76x10-4 2. 0.593 3. 3.32 4. 10.7 5.None of the abovearrow_forwardA. Complete and balance the reaction: HCI (aq) + H20 (0) --> ? B. Consider a 3.22x10M HCI (aq) solution. Calculate the [H30"), [OH], and pH of the solution. C. Enter the pH of the solution below. Show your work. Round the pH to the hundredths place. Tyne vour answerarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY