
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Which proton in each of the attached drugs is most acidic? THC is the active
component in marijuana, and ketoprofen is an anti-inflammatory agent.
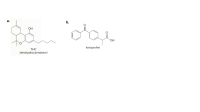
Transcribed Image Text:а.
b.
он
HO.
ketoprofen
THC
tetrahydrocannabinol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- F Below are four molecules that are pH sensitive: F O Structure A OH F O Structure OH Br O Br Structure C 1. Sort the given molecules in the increasing of pka values. OH 2. What was your choice for strongest acid? Explain your answer. Br Br Structure D OHarrow_forwardConsider the following molecules: он он ČI A Which is the stronger acid? Which structural effect led you to that answer? (If more than one effect is present, select the more significant effect)arrow_forwardAcid Conjugate base Acid H2CO3 HCO3 Base Strength Strength Increases Increases H2PO4 HPO,- (1) The acid with the smallest value of Ka is (A) H2CO3 (B) H2PO4 (1) The acid with the largest value of pKa is (A) H2CO3 (B) H2PO4arrow_forward
- 5. Use the following reaction to answer each part of this question: NaCN + OH HCN + iona ONa (a) Label the acid, base, conjugate acid, and conjugate base. (b) What is the pKa of the acid and of the conjugate acid? (c) What is the strongest acid in this reaction? (d) Does the equilibrium lie to the left (toward reactants) or to the right (toward products)?arrow_forwardThe Keq for the reaction: A + B ↔ AB is 2.03 What is the Keq for AB ↔ A + B Keq = ?arrow_forwardHCN +HPO4 2- CN-1+H2PO4-1 Which specie is conjugate acid?arrow_forward
- What does amphoteric mean? Group of answer choices The substance has a negligible conjugate base. The substance is neutral. The substance can donate either one or two protons. The substance can act either as a Bronsted acid or a Bronsted base in aqueous solution. The substance contains a carboxylic acid functional group.arrow_forwardplease explain answerarrow_forward5). Which of the following compound has lowest pKa? H3C (A) COOH F (B) COOH 6). What is the product nhtained from the full COOH (C) COOH (D) COOH (E)arrow_forward
- Given the following acid-base equilibria:HCOOH + H2O ---> H3O+ + HCOO-CH3NH3+ + H2O---> H3O+ + CH3NH2HSO4- + H2O---> H3O+ + SO42-Circle strongest acid and cross out the weakest.HCOOH HSO4- CH3NH3+Circle strongest base and cross out the weakest.HCOO- SO2- CH3NH2arrow_forwardWhat is the conjugate acid to HPO, ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY