
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![### Acidity and Basicity of Solutions
1. **Sodium Chloroacetate Solution**
- **Concentration:** \(0.180 \, \text{M}\)
- **Acid Dissociation Constant (\(K_a\))** of Chloroacetic Acid (\(\text{CICH}_2\text{COOH}\)): \(1.36 \times 10^{-3}\)
- **Hydronium Ion Concentration \([\text{H}_3\text{O}^+]\):** \( \boxed{\,}\) M
2. **Aniline Hydrochloride Solution**
- **Concentration:** \(3.90 \times 10^{-3} \, \text{M}\)
- **Base Dissociation Constant (\(K_b\))** of Aniline (\(\text{C}_6\text{H}_5\text{NH}_2\)): \(3.98 \times 10^{-10}\)
- **Hydronium Ion Concentration \([\text{H}_3\text{O}^+]\):** \( \boxed{\,}\) M
3. **Iodic Acid Solution**
- **Concentration:** \(0.210 \, \text{M}\)
- **Acid Dissociation Constant (\(K_a\))** of Iodic Acid (\(\text{HIO}_3\)): \(0.17\)
- **Hydronium Ion Concentration \([\text{H}_3\text{O}^+]\):** \( \boxed{\,}\) M
### Explanation
This section provides exercises for calculating the hydronium ion concentration \([\text{H}_3\text{O}^+]\) in solutions of varying strengths and chemical properties. Understanding such calculations is crucial in determining the acidity or basicity of a solution.
- **Part (a)** deals with a weak acid salt (sodium chloroacetate) whose \([\text{H}_3\text{O}^+]\) is determined by its \(K_a\).
- **Part (b)** requires converting a weak base constant (\(K_b\)) into its corresponding acid constant to find \([\text{H}_3\text{O}^+]\).
- **Part (c)** involves a strong acid (\(K_a\)](https://content.bartleby.com/qna-images/question/68a9b3d6-216d-4727-afb3-4b2a53419445/d63780c4-64a9-4d8a-a6db-956599dcc803/ezvtsyd_thumbnail.png)
Transcribed Image Text:### Acidity and Basicity of Solutions
1. **Sodium Chloroacetate Solution**
- **Concentration:** \(0.180 \, \text{M}\)
- **Acid Dissociation Constant (\(K_a\))** of Chloroacetic Acid (\(\text{CICH}_2\text{COOH}\)): \(1.36 \times 10^{-3}\)
- **Hydronium Ion Concentration \([\text{H}_3\text{O}^+]\):** \( \boxed{\,}\) M
2. **Aniline Hydrochloride Solution**
- **Concentration:** \(3.90 \times 10^{-3} \, \text{M}\)
- **Base Dissociation Constant (\(K_b\))** of Aniline (\(\text{C}_6\text{H}_5\text{NH}_2\)): \(3.98 \times 10^{-10}\)
- **Hydronium Ion Concentration \([\text{H}_3\text{O}^+]\):** \( \boxed{\,}\) M
3. **Iodic Acid Solution**
- **Concentration:** \(0.210 \, \text{M}\)
- **Acid Dissociation Constant (\(K_a\))** of Iodic Acid (\(\text{HIO}_3\)): \(0.17\)
- **Hydronium Ion Concentration \([\text{H}_3\text{O}^+]\):** \( \boxed{\,}\) M
### Explanation
This section provides exercises for calculating the hydronium ion concentration \([\text{H}_3\text{O}^+]\) in solutions of varying strengths and chemical properties. Understanding such calculations is crucial in determining the acidity or basicity of a solution.
- **Part (a)** deals with a weak acid salt (sodium chloroacetate) whose \([\text{H}_3\text{O}^+]\) is determined by its \(K_a\).
- **Part (b)** requires converting a weak base constant (\(K_b\)) into its corresponding acid constant to find \([\text{H}_3\text{O}^+]\).
- **Part (c)** involves a strong acid (\(K_a\)
Expert Solution
arrow_forward
Step 1: Describing the formula
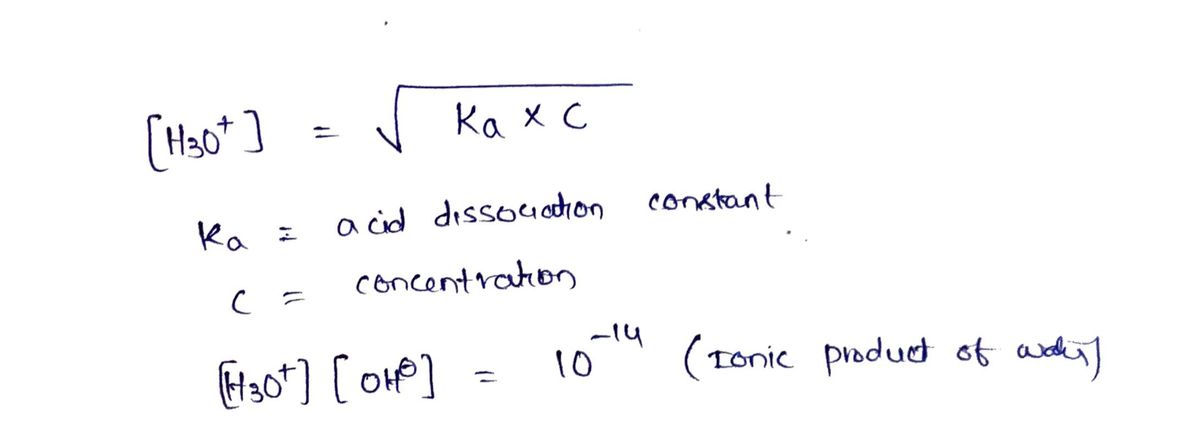
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A.) What is the pOH of an aqueous solution of 0.364 M nitric acid? B.) If 20.1 grams of an aqueous solution of aluminum nitrate, Al(NO3)3, contains 2.34 grams of aluminum nitrate, what is the percentage by mass of aluminum nitrate in the solution? C.) A sample of methane gas at a pressure of 0.638 atm and a temperature of 21.8 °C, occupies a volume of 11.1 liters. If the gas is compressed at constant temperature to a volume of 2.91 liters, the pressure of the gas sample will be__________ atm. ( fill in the blank)arrow_forwardWhat mass of sodium acetate must be added to 435 mL of water to give a solution with pOH = 4.65? Kb of acetate is 5.68 x 10-10.arrow_forwardWhat is the pH of 0.10 bicarbonate (HCO3-) solution? Kb=2.4E-8. X= ? [H30+] = ? pH = ? %3D %3D What is the pH of a .80 M hydrofluoric acid (HF) solution? Ka= 6.6E-4. X= ? pH= ?arrow_forward
- Each row of the table below describes an aqueous solution at about 25 °C. Complete the table. That is, fill in any missing entries in the second and third columns. Be sure each entry you write includes the correct number of significant digits. [1,0'] solution pH x10 11 A 5.9 X 10 mol/L В ||mol/L 2.25 7 2.0 x 10 mol/Larrow_forwardOxygen (O2) is necessary for cellular respiration; it must be transported from the lungs to tissues through the bloodstream. However, O2 is not very soluble in water. The Keq of oxygen dissolving at 300.0 K is 7.64 x 10-4 and the Keq at 320.0 K is 5.23 x 10-ª. Calculate the AH(dissolve) for oxygen solubility in water, in kJ/ mol. kJ/mol 1 3 4 C 7 8 +/- х 100 2. LOarrow_forward5. What amount of solid NaOH must be added to 1.0 L of a 0.12 M H2CO3 solution to produce a solution with [H+]= 3.1x10-11 M ? There is no significant volume change as the result of the addition of the solid.arrow_forward
- What is [H3O+ ] (in M) in a solution of 0.063 M HCNO and 0.035 M NaCNO? (Assume Kw = 1.01 ✕ 10−14.) HCNO(aq) + H2O(l) H3O+(aq) + CNO−(aq) Ka = 3.5 ✕ 10−4arrow_forwardEach row of the table below describes an aqueous solution at about 25 °C. Complete the table. That is, fill in any missing entries in the second and third columns. Be sure each entry you write includes the correct number of significant digits. [1,0"] solution pH x10 olo -8 A 1.5 X 10 mol/L Ar В ||mol/L 2.24 - 11 mol/L 2.1 X 10 Carrow_forwardConsider the reaction of 66.5 mL of 0.310 M NaC₇H₅O₂ with 50.0 mL of 0.245 M HBr. (Ka of HC₇H₅O₂ = 6.3 x 10⁻⁵) a)Write the net ionic equation for the reaction that takes place. (In terms of C, H2O, H3O+, O, H, H+, OH-) b) How many moles of C7H5O2- would be present before the reaction takes place? c) How many moles of H+ would be present before the reaction takes place? d)What species would be left in the beaker after the reaction goes to completion? e) How many moles of C7H5O2- would be left in the beaker after the reaction goes to completion? f) How many moles of HC7H5O2 would have been produced after the reaction goes to completion? g) What would be the pH of this soultion after the reaction goes to completion?arrow_forward
- The weak acid, chloroacetic acid, is a useful reagent in organic synthesis owing to a reactive C-CI bond. It has a K. =1.40x103.arrow_forwardP Please don't provide the handwriting solutionarrow_forward+ In an aqueous solution at 25 °C, if [H₂O*] = 1.1 × 104 M, then [OH-] is: 1 4 7 +/- 2 5 8 | M 3 6 9 0 X C x 100arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY