
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
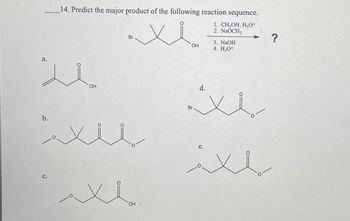
Transcribed Image Text:a.
.14. Predict the major product of the following reaction sequence.
منا.
Br
OH
1. CH3OH, H3O+
2. NaOCH
3. NaOH
4. H3O+
OH
منذ
للام
OH
?
لللا
e.
سلام
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 18 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Reactions. Suggest reagents for steps a-d in the following synthetic sequence CH3 CH3 CH3 b. a. HO CH3 CH3 но, LDA or strong base CH3 CH3 CH .CN с. но. CN d. CH3 H3C CN H3C CN Draw the major product of the following reactions H3C. 120 °C CN (no need to show stereochemistry) H2 (gas) cat. Pd (show relative stereochemistry)arrow_forward2. In the synthesis of peptides, carboxylic acids are condensed with amines in the present of a reagent such as dicyclohexylcarbodiimide (DCC) [Section 25.6]. a. Propose a mechanism for the following, using curved arrow notation. H₂N. Мон glycine H₂N. + OH Дон DCC H₂N. .OH OH H glycine gly-glyarrow_forwardPropose a curved arrow mechanism to account for the formation of compound C.arrow_forward
- Hw.129.arrow_forwardThe below synthesis was designed using the Organic Chemistry Roadmaps in the appendix of your textbook. compound a m compound b q Reagents a. HCI b. HBr c. H₂O, H₂SO4 d. Br2 Cl₂ H₂, Pd compound c e. f. g. h. i. j. Br₂, H₂O Cl₂, H₂O OsO4 then NaHSO3 k. I. m. 2 equivalents of NaNH₂ n. H₂, Lindlar's catalyst O. p. Hg(OAc)2, H₂O then NaBH4 BH3 then H₂O₂, NaOH O3 then (CH3)2S In this synthesis, reagents from the table (shown in blue) are used to carry out the indicated reactions. In the box below, draw the structure of compound b. q. r. Na/NH3 H₂SO4, HgSO4 (sia)₂BH then H₂O₂, NaOH 1 equivalent of NaNH2 • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • Consider E/Z stereochemistry of alkenes. ● If more than one structure fits the description, draw them all. ● If a compound is formed more than once, add another sketcher and draw it again. • If the reaction produces a racemic mixture, draw both stereoisomers. Separate structures with + signs from the drop-down…arrow_forwardPredict the major product for the following reaction. A. مسلم مة في ملية کی B. E. NaOH/H₂O A C.arrow_forward
- 4. There are six steps in the mechanism below. Identify the basic moves in this complicat ed mechanism – one we will see next semester when we discuss carbonyl compounds. The point is that even complicated mechanisms can be broken down into simple terms and movements we recognize from the beginning of organic chemistry. Н. Н A. но B. н но он D. Он E. H. Н F. :o: Онarrow_forwardGive Clear Detailed Solution with explanation needed..don't give Handwritten answerarrow_forwardChoose the reagents that can be used to perform the following transformation: Select one: a. "OH, H₂O ...OH e. OH b. H3O+ X c. Na₂Cr₂O7, H₂SO4 d. KMnO4, OH Both -OH, H₂O and H3O+ (separately) will work.arrow_forward
- Select reagents from the following table to bring about this conversion. a. HOCH₂ CH₂ OH, H+ 1. Os04 2. NaHSO3 RCO3 H H3O+ NaBH₁ b. (Enter your result as a string of letters, in the order that you wish to use the reagents, i.e. ade.) C. d. e. LOH f. g. HO dihydropyran, H+ 1. BH3 2. NaOH, H₂O, H2₂ O2 OHarrow_forward5. Propose a synthesis of each of the following compounds using the indicated starting material. You may use any organic compounds, inorganic compounds, organometallic compounds, or solvents of your choice. Do not show any reactive intermediates, mechanisms, or transition states, but be sure to show each isolable compound along your synthetic route. a. b. C. Ph + PPh3 Cl mylom Ph. CH3 steps steps steps H3C. H3C Ph CH3 CH3 Ph OH ÕH Ph (racemic) 4arrow_forwardGive detailed Solution with explanation needed...don't give Handwritten answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY