
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
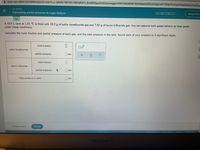
Transcribed Image Text:A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQİHQRDYV_6Ux63SypJXz0Coxvwqgg4JkWI7tMn8ANh-BzK5qlesVjsfAuQzXgmoMTWNg1Xu4cyjdVpsjQib5plVDqEPg
O GASES
Calculating partial pressure in a gas mixture
Jacqueline
A 10.0 L tank at 1.41 °C is filled with 19.2 g of sulfur hexafluoride gas and 7.63 g of boron trifluoride gas. You can assume both gases behave as ideal gases
under these conditions.
Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits.
mole fraction:
sulfur hexafluoride
partial pressure:
|atm
mole fraction:
boron trifluoride
partial pressure:
| atm
Total pressure in tank:
atm
Explanation
Check
O2021 McGraw-Hill Education All Rights Reserved Terms of Use Privacy Accessibility
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Need help with this questionarrow_forward-> https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9QI15ULF571w... A to tho subject) - maryhan X M <3- mary.hamilton@s X To 3 1 O ADVANCED GENERAL CHEMISTRY Calculating an equilibrium constant from an equilibrium.. 3/5 Hydrogen and chlorine react to form hydrogen chloride, like this: H,(g) + Cl,(g) → 2 HCl(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following composition: compound pressure at equilibrium H2 62.7 atm Cl 35.7 atm HCl 85.0 atm Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. d. K D x10 Eſplanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Usel Privacy Center | Acce P W F7 F8 F9 F10 F11 F12 PriScr risert Delete FA FS F6arrow_forward3. 11 100 TRANSMETTANCE D C8H9CIO 4000 10 HSP-01-151 9 3000 8 2:2 7 2000 6 : 5 ppm 2 NAVENUMBERal 4 3 1500 : Vala 2 1000 3 1 0 500 200 180 COS-04-715 DEPT-90 DEPT-135 160 140 120 بلد لد 100 ppm 80 60 40 20 0arrow_forward
- A ALEKS-Lara Althawadi - Lear X G 3.0 sig figs - Google Search X G 0.011 sig figs - Google Search x + www-awu.aleks.com/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3JH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInL27j0m-BbhK772LRC-WPOIJB064JOgeD... ✰ 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E.... Reading Schedule 19.6 Reduction Po... Y SOLUTION: The le Math 115 W-S Fall.... C Solubility and.... SC O ACIDS AND BASES Calculating the pH at equivalence of a titration A chemist titrates 160.0 mL of a 0.5839M benzoic acid (HC,H,CO₂) solution with 0.4219M KOH solution at 25 °C. Calculate the pH at equivalence. The p K of benzoic acid is 4.20. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = 0 Explanation Check Methods for Measuring the pH of an Aqueous Slide 28 of 104 Notes X Click to add notes 19 Comments S P DA :: R 5 + 63% 53 T 6 G 00000 3/5 I'm B h. Get…arrow_forwardCh3 interconvertingarrow_forwardALEKS-Alasia Fuqua - Learn ||| www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBjuJnuCDtT6kRBabGFF3MoAkZ_UVx0N202gq0GbTcR2WLHPX1Jb033xbT1iMAMuscRihtTHLJczaStvK2zINR T3W14vbTy2108w2Qv = F (SPAN-101-001) Beginning Spar X 11 O ACIDS AND BASES Predicting the relative acidity of binary acids Enter the chemical formula of a binary molecular compound of hydrogen and a Group 4A element that can reasonably be expected to be more acidic in aqueous solution than SiH, e.g. have a larger K Explanation VHL Central | Sections Check Do X O Alasia © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility Jan 23 ? 610 dla B 7:178arrow_forward
- MyCSU-Columbus State Univers X M Inbox (1) - bailes_amber@colum X D2L Quiz 1- Intro Resrch Meth in CJ K ← → CO 03 Email MGmail X A ALEKS-Amber Bailes - Learn X + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJgXZp57itWHhRgilODc5MqvhZbKYx2-U-03700TYd4ABbH6KAJLiK5JfbHdk1X12WQ... LE YouTube Maps MyCSU-Columbus... B Homepage - Georg... Microsoft Office Ho... BLesson 2 Discussion... III MyMidland Mortga... CHEMICAL REACTIONS Solving for a reactant using a chemical equation 3/5 Green plants use light from the Sun to drive photosynthesis. Photosynthesis is a chemical reaction in which water (H₂O) and carbon dioxide (CO₂) chemically react to form the simple sugar glucose (C6H₁2O6) and oxygen gas (0₂). 12 What mass of carbon dioxide is consumed by the reaction of 3.6 g of water? Be sure your answer has the correct number of significant digits. g x10 Ś ? Explanation Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce 78°F O Et C ||| Check Type here to search X a no…arrow_forwardAssistive Technologies for S X O MYCSU - Columbus State UX Link to AL https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-JgXZp57itWHhRgilODc5MqvhZbKYx2-U-038xjApUucKTA610SL O MEASUREMENT AND MATTER Predicting the formula of ionic compounds with commo.. Write the empirical formula for at least four ionic compounds that could be formed from the following ions: CH,CO,, NH", Fe*, Cro IIarrow_forwardIqua - Learn X + www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBjuJnuCDtT6kRBabGFF3MoAkZ_UVXON202gNa615nmsU8E-ZOOTYSonoqYtZeiTnd410r70if0aJzborkyz0qYYZASKGXo?10Bw7QYjlba... ||| O CHEMICAL REACTIONS Using a chemical equation to find moles of product from moles... Ammonium phosphate ((NH₂), PO4) is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid (H₂PO) with liquid ammonia. Calculate the moles of phosphoric acid needed to produce 1.30 mol of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. 0 Explanation Check X 0 x10 0.0 3/5 5 Alasia dh E ? 0 12:36 09arrow_forward
- KS - Alec Nema - Learn + A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-lvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym9ptTdXEBuyh4nRbErwikmldvGYg7DAf2EO_jCH3o... ☆ Sprouts Academy. Online Tutoring C 400 Request Heade. Q Weather & Soil CH.. O ADVANCED MATERIAL Using solubility to calculate solute mass or solution volume Alec The solubility in water of ionic compound X is measured and found to be 0.143 g at 10. °C. Calculate the volume of a saturated solution of Xin water that ml would contain 210. mg of X at this temperature. Be sure your answer has the correct unit symbol and 3 significant digits. Explanation Check O 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy| Accessibilit 4:20 11/25 35 O Type here to search DELLarrow_forwardAnnouncements- JMATH1010 E Announcements - J-MATH1010 A ALEKS - Antionette Thomas - Le x i www-awu.aleks.com/aleksogi/x/Isl.exe/10_u-IgNslkr7j8P3jH-lIJQhIJODya094wj2gIJskib9agPtGFIZ2D9vGhtDPlvknuFF-VYYIW_FaqZsAPoIPVZF3RSL9İ5ekYIJjK. O DECIMALS, PROPORTIONS, AND PERCENTS Word problem on proportions: Problem type 2 DOO OO 3/5 Antionette Espar A certain drug is made from only two ingredients: compound A and compound B. There are 4 milliliters of compound A used for every 3 milliliters of compound B. If a chemist wants to make 553 milliliters of the drug, how many milliliters of compound B are needed? milliliters of compound B 图 Enplanation Checkarrow_forwardA ALEKS- Allie Fleming - Learn X + s.com/alekscgi/x/lsl.exe/10_u-IgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9QIZnmclTUEg1JWvSyM1tTaqBJUR5NSUOCIG_6ulnLcZ71D4j2Qcp = Organic Functional Groups Understanding common names of carboxylic acids and derivatives In the drawing area below, draw the skeletal ("line") structures of propionic acid and potassium propionate. You can draw the two molecules in any arrangement you like, so long as they don't touch. Explanation $ 4 % Check 5 Click and drag to start drawing a structure. NA J- ^ Q Search (0) 6 X ♫+ & 7 fa X KAA RTY U * 8 DII 110 DDI 9 X 0:0 W 3 fm © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ODOO 0/5 10 V insert P prt sc + =arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY