Question
thumb_up100%
I got 0.0104891861 Nm for my answer and don't see how that is incorrect.
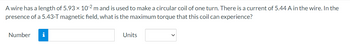
Transcribed Image Text:A wire has a length of 5.93 × 10-2 m and is used to make a circular coil of one turn. There is a current of 5.44 A in the wire. In the
presence of a 5.43-T magnetic field, what is the maximum torque that this coil can experience?
Number i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions
- Scientists are conducting an experiment to determine if their hypothesis that a certain constant in the universe is 1.65. the uncertainties in the experiment result in a relative uncertainty of no more than 2%. After several experimental trials, the scientists obtained an average value of 1.7 for the constant. What can be said about the scientists hypothesis? Hint calculate the percent error and compare it to the relative uncertainty.arrow_forwardUse the Widmark Equation, , to solve this question. Recall that r = 0.68 for men and r = 0.55 for women. B = percentage of BACN = number of “standard drinks” (A standard drink is one -ounce beer, one -ounce glass of wine, or one -ounce shot of liquor.) N should be at least one.W = weight in poundsr = the distribution rate for alcohol through the body (this value is a constant), for males and for femalest = number of hours since the first drink A male student had four glasses of wine at a party. He weighs 172 pounds. How long will it take before his BAC falls to 0.05? 3.14 hours 3.57 hours 1.00 hours 4.29 hoursarrow_forwardThe elliptical galaxy NGC 4889 is the largest galaxy in the Coma Cluster (shown in the image below taken by the Hubble Space Telescope). After analysing the spectrum of NGC 4889, an astronomer identifies a spectral line as being CaII (singly ionised Calcium) with a measured wavelength of 401.8 nm. The true, rest wavelength of this spectral line, measured in a lab, is 393.3 nm. Using a Hubble constant of ?0 = 70 km/s/Mpc, find the distance to this galaxy cluster. Give your answer in megaparsecs and in light-years.arrow_forward
- Determine the distance between the electron and proton in an atom if the potential energy UU of the electron is 11 eV (electronvolt, 1 eV =1.6×10−19=1.6×10−19 J). Give your answer in Angstrom (1 A = 10-10 m).arrow_forwardYour trusty 1 milliwatt He-Ne laser beam has a wavelength 1 = 632.8 nm |(the correct wavelength). Perform the following calculations, or answer the following questions about your prized possession: 1) calculate the energy, E, of the He-Ne laser beam in electron volts (eV); 2) to your eyes, what color does this laser beam's wavelength correspond to (in air)?; 3) If this laser beam is focused into a 1 carat diamond, with index of refraction n = 2.42, calculate the speed of the He-Ne laser beam, as you check the quality of your purchase at Zale's Diamonds, with your best girl.arrow_forwardWhat is the Energy, in eV, of a light wave with frequency of 6 * 10^15 Hz?arrow_forward
arrow_back_ios
arrow_forward_ios