
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Can you show me how to do this type of question please?
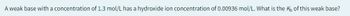
Transcribed Image Text:A weak base with a concentration of 1.3 mol/L has a hydroxide ion concentration of 0.00936 mol/L. What is the Kb of this weak base?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the functional group of propanoic acid? How do we know if it's soluble in water or not using the flowchart that is attached. which test should be performed using the flowchart. Is propanoic acid a ketone, aldehyde, ester or alcohol and how did you know?what would be the most efficient series of solubility and functional group tests that would identify the functional group? Also what could be the sources of error in this experiment? What could occur that would give false positives, false negatives, or incorrect interpretations?arrow_forward||| O ORGANIC FUNCTIONAL GROUPS Predicting the products of symmetric alcohol dehydration Predict the missing product of this organic reaction: H+ A OH planation + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. Click and drag to start drawing a structure. Check www-awu.alekarrow_forwardNonearrow_forward
- Predict te organic product that forms in the reaction below: H Xy+ OH H+ OH H+ Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the product. In the drawing area below, draw the skeletal ("line") structure of the missing organic product X. Click and drag to start drawing a structure.arrow_forwardxxxixarrow_forwardGive the wet chemical tests (#1-6) that work for ALDEHYDES only.arrow_forward
- HOW AIR FRESHENERS WORK Some air fresheners just mask bad smells, while others claim to eliminate odors completely. Here. we review the different types of compounds found in air fresheners and how they combat stench. FRAGRANCES ODOR TRAPPING Air freshener aroma compounds mask bad smells. They include terpenes such as limonene and a-pinene. Some people have expressed concern that these can react with ozone to produce formaldehyde, a carcinogen. ODOR NEUTRALIZING Some air fresheners use organic acids, which can react with smelly compounds to break them down to more benign molecules. CITRIC ACID MALEIC ACID LIMONENE E a-PINENE HO но. PERIODIC GRAPHICS OH OH OH OH air freshener Cyclodextrins are ring-shaped molecules made from cornstarch. Odor molecules get caged within cyclodextrin's cavity, stopping them from reaching your nose. HO HO O HO LOH OH OH OH ODOR MOLECULES OH HO OH OH OH O HO HO HO_ B-CYCLODEXTRIN HO OH HYDROPHOBIC HYDROPHILIC EXTERIOR Hydrophobic odor molecules get trapped in…arrow_forwardCan you please give an explanation for question 2( A to J)? Thank youarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY