
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
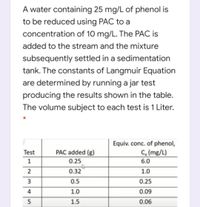
Transcribed Image Text:A water containing 25 mg/L of phenol is
to be reduced using PAC to a
concentration of 10 mg/L. The PAC is
added to the stream and the mixture
subsequently settled in a sedimentation
tank. The constants of Langmuir Equation
are determined by running a jar test
producing the results shown in the table.
The volume subject to each test is 1 Liter.
Equiv. conc. of phenol,
C, (mg/L)
PAC added (g)
0.25
Test
1
6.0
0.32
1.0
3
0.5
0.25
4
1.0
0.09
5
1.5
0.06
2.

Transcribed Image Text:If the water concentration is
reduced from 25 mg/L of phenol to
a concentration of 10 mg/L, what is
the adsorption capacity of the
PAC? *
Your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Liquid benzene and liquid n-hexane are blended to form a product stream flowing at a rate of 2,600 . Ibm h n-hexane Product stream Mixer benzene The density of the product stream is measured and found to be 0.75 mL' What is the volumetric flow rate of the product stream in ft3 hour Iba ? What is the mass flow rate of benzene in harrow_forwardSolve 2 pleasearrow_forward7-31. In the air dryer illustrated in Figure 7.31, part of the effluent air stream is to be recycled in an effort to control the inlet humidity. The solids entering the dryer (Stream #3) contain 20 % water on a mass basis and the mass flow rate of the wet solids entering the dryer is 1000 lb/h. The dried solids (stream 4) are to contain a maximum of 5 % water on a mass basis. The partial pressure of water vapor in the fresh air entering the system (Stream #1) is equivalent to 10 mm Hg and the partial pressure in the air leaving the dryer (Stream #5) must not exceed 200 mm Hg. In this particular problem the flow rate of the recycle stream (stream #6) is to be regulated so that the partial pressure of water vapor in the air entering the dryer is equivalent to 50 mm Hg. For this condition, calculate the total molar flow rate of fresh air entering the system (Stream #1) and the total molar flow rate of the recycle stream (Stream #6). Assume that the process operates at atmospheric pressure…arrow_forward
- A sample of 200 mL of water was collected from a river. From this sample, 5 mL was diluted to 1-L. A BOD bottle (which is 300 mL) was filled with this diluted water and aerated. The dissolved oxygen content of the diluted sample was 7.8 mg/L initially. After 5 days, the dissolved oxygen content had dropped to 4.8 mg/L. What is BOD5 of the river water? 600 mg/L O 15 mg/L 10 mg/L O 3mg/L 2 mg/Larrow_forwardA compound dissolves in water at a rate proportional to the product of the amount undissolved and the difference between the concentration in a saturated solution and the concentration in the actual solution at any time. A saturated solution of compound contains 40 g/100 g H2O. In a test run starting with 20 kg of undissolved compound in 100 kg of pure compound is found that 5 kg is dissolved in 3 hr. If the test continues, how many kilograms of compound will remain undissolved after 7 hr? Assume that the system is isothermal. Solution containing undissolved compound Figure 4arrow_forwardIn the aeration of wastewater, liquid-gas contact systems are designed to raise the concentration towardequilibrium levels. This goal is accomplished by dispersing air bubbles into the water. An aqueous solution,initially containing 2 x 10 -3 kg O2 /100 kg H2O is brought into contact with a large volume of ordinary air at 293K and a total pressure of 1.013 x 10 5 Pa. At 293 K, the Henry’s law constant for the oxygen–water systemequals 4.06 x 10 9 Pa/mol fraction of oxygen in the liquid. a.) Will the solution gain or lose oxygen? b.) What will be the concentration of oxygen in the final equilibrium solution in kg O2 per 100 kg H2O?arrow_forward
- A tank contains certain volume of fresh water .A stream of brine containing 2 gm/lit of salt fed into tank at a rate 4lit/min, liquid flows from the tank at a rate 3lit/min. if the tank is well agitated and the salt concentration in the tank attains a value which is 50% j ẩjet brinej ẩẩenẩtration in 40 minute find out the initial volume fresh water in the tank.arrow_forward10. A container is separated into two halves by a membrane. Your lab partner assistant, Thurmond, is supposed to place a MgCl2 solution on side 1 and a NaCl solution on side 2. He is then supposed to measure the rate of water movement across the membrane. Unfortunately, Thurmond is not very good about keeping complete lab notes and he has forgotten to write down some data and calculations. Using your knowledge of osmosis, complete the following table. Temperature is 15°C and the hydraulic conductivity for the membrane is 0.4 ml/atm sec. MgCl2 Concentration on Side 1 NaCl Concentration on Side 2 80 mM Osmolarity on Side 1 Osmolarity on Side 2 Difference in osmotic pressure 60 mosM across the membrane Jy 0.95 ml/sec Reflection coefficient (ơ) Direction of water movementarrow_forwardComplete the question by hand calculation(please show every step):-Do the mass balance, and find the flow rate of the vapour and liquid outlets of the last separator and purge stream and recycle stream flow rate for the following question by HAND CALCULATION:- Methanol can be produced from natural gas(syngas gas)the following syngas gas (feed) has the following conditions and composition 915 kgmole/hour Temperature 131.7 ºCPressure 1187 psiaComponent Composition (mol%)Carbon Monoxide 15.63Carbon Dioxide 7.93Hydrogen 73.31Nitrogen 0.33Methane 2.61Water 0.19TOTAL 100.00How the proccess flow diagram is attached to this question.Details of the process:- Firstly feed is mixed with the recycle stream using a mixer Output of the mixer is put into the reactorwhere the following two reactions occur:-CO2 + 3H2 <--> CH3OH + H2OANDCO + 2H2 <--> CH3OH Reactor output is cooled to from 246.3 degrees celcius to 37.78 degrees using a cooler(using 4.229e+007 of heatflow) Then the cooled…arrow_forward
- Calculate the rate of absorption of ammonia from a dilute gaseous mixture with nitrogen by water in a wetted-wall column. The followingexperimental data are available: Inside diameter of column = 25 mmAverage Gas velocity = 250 cm/sGas Temperature = 25°CTotal Pressure = 1 atm At a certain point in the column, the partial pressure of ammonia in N2is 30 mm Hg and the mole fraction of NH3 in N2 at the gas-side of the interface is 0.005.arrow_forwardニイ91 A mixture of n-heptane (03%), Benzene(45%), xylene(50%) and Ethyl-Benzene is fed into a distillation column operated Problem 1: at one bar. The feed Jatm is saturated liquid. If 90% of Benzene and 6% of xylene appears in distillate calculate the approximate distillate and bottoms flows and compositions assuming 100 moles of feed Problem 2: Using your results in problem 1 and assuming 4= 2 Alfa(bZ/Tol)=2.0 R=1.3 Rmin calculate: ト Nmin ji- Rmin assuming pseudo binary separation iii- iv- N using Gilliand correlation V- Number of theoretical stages in both sections vi- Optimum feed locationarrow_forwardA gas stream that conatins 1.0 mole% CO2 flows through a pipeline. 50 kg of CO2 per minute is injected into the line. A sample of the gas is drawn from a point in the line 150 meters downstream of the injection point and found to contain 2 mole% CO2. Determine the flow rate of a gas stream upstream of the injection point.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The