
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:O Attempt 1
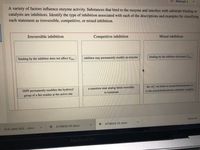
A variety of factors influence enzyme activity. Substances that bind to the enzyme and interfere with substrate binding or
catalysis are inhibitors. Identify the type of inhibition associated with each of the descriptions and examples by classifying
each statement as irreversible, competitive, or mixed inhibition.
Irreversible inhibition
Competitive inhibition
Mixed inhibition
binding by the inhibitor does not affect Vmax
inhibitor may permanently modify an enzyme
binding by the inhibitor decreases Vmas
the Alt ion binds to acetylcholinesterase or
to the acetylcholinesterase-substrate complex
DIPF permanently modifies the hydroxyl
a transition state analog binds reversibly
to isomerase
group of a Ser residue at the active site
Show All
W-
5179933 (3).docx
W-
5179933 (4).docx
PCR-MINI RES...docx
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- List the local effectors of enzyme kinetics and give an example for one of them.arrow_forwardPlease handraw this graph with all the necessary detailed information: Imagine that I text enzyme rate for four different temperatures: 10 degrees celsius, 20 degrees celsisus, 30 degree celsius, and 40 degree celsius, in separate tubes. The enzyme appears to work faster as temperature increases, but completely ceases activity at 40 degrees celcius. Sketch a graph to show this outcome, but here you will graph product formation (nmoles/mL) vs. time (minutes). The graph should be 4 lines and HANDDRAWN. Include a legend if necessary. You do not need precise quantitivate values, but most show the correct trends on the graph.arrow_forwardPlease name the differences between competitive and noncompetitive inhibition.arrow_forward
- Identify the different factors influencing enzymes including inhibitors, pH, and temperature.arrow_forwardDraw the graphs representing activity behavior for each uncompetitive Inhibition, non-competitive Inhibition, and competitive Inhibition. Please use a ruler, draw, photograph, and insert the image in this document. Label x and y axes, Km and Vmax.arrow_forwardDescribe the specific multi-enzyme example discussed in class of how enzyme activity can be altered by changing its substrate to product ratio.arrow_forward
- (a) You are given the following experimental measurements for the effects of an inhibitor on the initial velocity of an enzymatic reaction. Plot the data and determine what type of inhibition is being observed. Label all axes. [S] (MM) Vo (mM • Vo with I present (mM ⚫s-¹) 1 2.8 3 6.0 1.0 2.5 4 7.0 3.1 8 9.9 12 11.5 4.8 5.7 (b) Briefly explain how varying [S] can be used to determine whether an inhibitor is uncompetitive or competitive. Be sure to include in your explanation why this method would work.arrow_forwardThe catalytic activity of enzymes depends on the presence of appropriate environmental conditions. Pepsin is a digestive enzyme found in the stomach and facilitates the digestion of large proteins. If pepsin is removed from this acidic environment of the stomach and is instead placed in a more basic environment, it will cease to function. Describe the specific effect that a change in environmental pH will have on pepsin and explain how this change can lead to inhibition of its catalytic activity. Respond in 4 to 6 complete sentences.arrow_forwardOne of the hallmarks of competitive inhibition is that there is constant competition betweenthe substrate and the inhibitor for binding to the enzyme active site.a) If [inhibitor] >> [substrate], which compound “wins” (i.e., occupies the active site a greaterpercentage of the time)?b) If [substrate] >> [inhibitor], which compound “wins” (i.e., occupies the active site a greaterpercentage of the time)?arrow_forward
- What is the relative inhibition of an enzyme by a competitive inhibitor at [S] = KS and [I] = KI?arrow_forwardAn inhibitor was added to an enzyme and the expected rate of the reaction was not detected and the substrate was not utilized at all. This inhibitor is (choose one answer only): Is un-competitive, meaning the inhibitor binds to a site near the active site. Is competitive, meaning the inhibitor binds directly to the same active site as the subtrate. Is non-competitive, meaning the inhibitor binds to site other than the active site as the subtrate. Is irreversible, meaning the inhibitor binds covalently to the enzyme keeping the enzyme inactive permanently.arrow_forwardWhich of the following are characteristics of catabolic metabolic pathways? (Choose all correct answers). Overall oxidation of initial substrates so redox reactions involve use of NAD+ as oxidizing agent, converted to NADH. Large initial substrates are broken down to smaller final products. Overall reduction of intial substrates so redox reactions involve use of NADPH as reducing agent, converted to NADP+. The pathways are overall exergonic (negative delta G). Net production of ATP as a result of the pathway. Essentially irreversiblearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON