
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
![a Use the rules for logarithms and exponents to solve for [H*] in terms of pH.
[H*] = |10PH
b If pH = 9.15 then
[H*] =[
|M](https://content.bartleby.com/qna-images/question/82f6d2bb-9c92-4d9c-a063-7cd77d3658c5/a18506cc-b106-4d19-98b1-ff6b063850de/dy5816_thumbnail.png)
Transcribed Image Text:a Use the rules for logarithms and exponents to solve for [H*] in terms of pH.
[H*] = |10PH
b If pH = 9.15 then
[H*] =[
|M
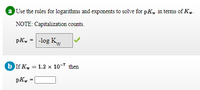
Transcribed Image Text:a Use the rules for logarithms and exponents to solve for pK, in terms of K..
NOTE: Capitalization counts.
pK, = -log Kw
b If K, = 1.2 x 10-7 then
pKw
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Find du=du (T,V) in terms OF measurable properties (P-V-T). Show the mathematical derivation.arrow_forwardUsing Maxwell's relations, find the equivalent expressions for the following. (a) ()s (b) ()s (c) (#)v (d) ()r Goal: Use Maxwell's relations to find the equivalent expressions. Recall: Since U, H, F and G are analytic functions, 3Y8Z 21.2. represents U, H, F or G and Y represents a thermodynamic variable T, P, V, N, or S. where Xarrow_forwardA rectangular block below of height L and horizontal cross-sectional area A floats at the interface between two immiscible liquids: Derive a formula for block density(ρb), in terms of the fluid densities ρ1 and ρ2, heights h0 and h1, and the cross-sectional area A. (Note, all variables may not appear in the final result).arrow_forward
- choose the correct answerarrow_forwardBegin from total differential equation (Exact DE). Substitute the measurable or P, T,V terms to nonmeasurable or the U, H,G,A,S terms. derive this equation: to: dH = H = H (T, P) (3FF) ƏT (OP) dF dP ӘР T dT + + + [V - Tor] ]d² dP dH = CpdT + V-Tarrow_forwardB)Draw the root locus for the following system: R(s) + K S(S+1)(S²+4S+13) C(s)arrow_forward
- The relative volatility of a binary mixture used for fractionation can bearrow_forwardes the first and second laws of thermodynamics to produce 10:15 LTE Today Edit 9:56 AM tap ), 4. The Third Law of Thermodynamics (a) provides a criterion for spontaneity (b) provides a criterion for spontaneity (c) establishes standard pressure (d) isolated systems constant T and V I equations of themodynamics (e) provides an absolute value for entropy at absolute zero. - i s ar The Third Law of Themadynamce provides a crterion for spontaneity for isolated s a enterion for spontanety at constant compes ot and aocond in of temade ndamental eguations of themodynamics provides an abolute value for entroy at absokarrow_forwardMethod of Analysis Solve y′′ − 4y′ + 3y = et/(1 + et) using the method of variation of parameters.arrow_forward
- 8.arrow_forwardQ1/Complete the following table for H2O: T (°C) Р (кра) v (m³ /kg) Phase description 60 4.131 300 Saturated liquid 250 200 150 1000 T (°C) Р (кра) u (kJ/kg) Phase description 20 5000 150 631.68 225 2000 30 Saturated liquid 300 2600 T (°C) Р (кра) h (kJ/kg) Phase description 500 200 175 486.99 55 600 400 4000 255 Saturated vapor T (°C) P (kpa) v (m³ /kg) X, if applicable Phase description 100 1000 130 0.00107 550 0.75 750 0.2556 150 75arrow_forwardchoose the correct answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The