
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Iga
86°F
Sunny
2
A typical reading of the air pressure in Rochester, New York,
is around 30.03 in. Hg. What is this reading in atmospheres?
(1 atm = 29.9 in. Hg)
W
O 1.004 atm
O 0.9957 atm
O 0.03951 atm
O 897.9 atm
#
3
*
E
f4
$
101
4
R
%
5
T
f6
O)
6
4-
&
Y
7
U
+
hp
8
9
Expert Solution
arrow_forward
Step 1
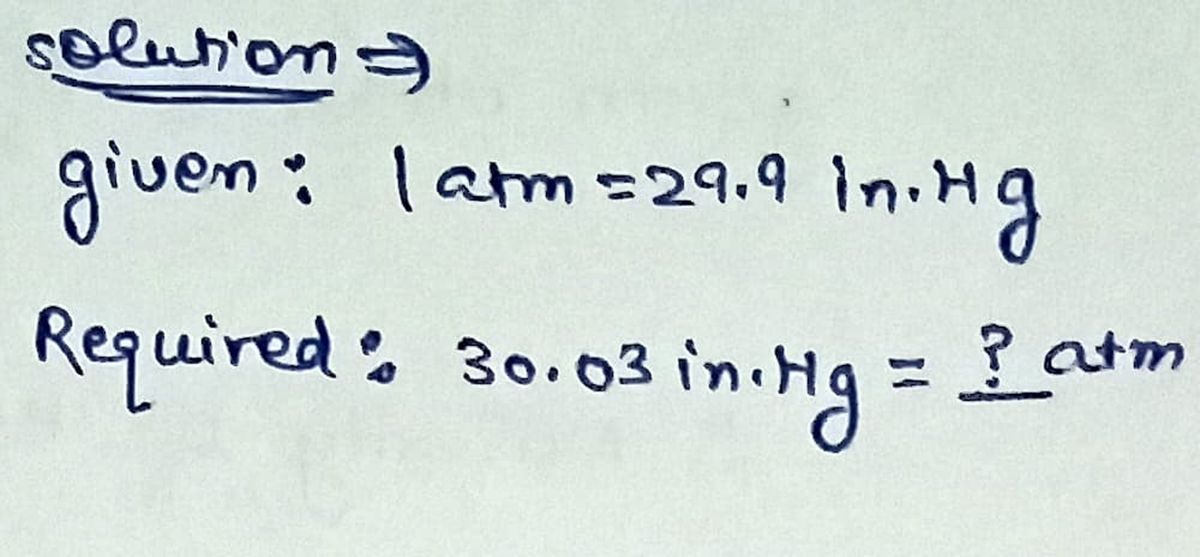
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You fill a balloon with 1.15 L of air at 22°C and 0.850 atm pressure. You then take this balloon to a new location where the temper- ature is 34°C and you find that the volume has decreased to 0.963 L. What is the new pressure inside the balloon? 1. 0.980 atm 2. 0.905 atm 3. 0.975 atm 4. 1.06 atmarrow_forwardThe diagram below shows a gas with an initial pressure of 4.0 atm in a cylinder at a constant temperature.The gas expands inside the cylinder and pushes the piston up. What is the final pressure of the gas after the expansion?arrow_forwardA 1.20 L weather balloon on the ground has a temperature of 25.0°C and is at atmospheric pressure (1.00 atm). When it rises to an elevation where the pressure is 0.710 atm, then the new volume is 1.80 L. What is the temperature (in °C) of the air at this elevation?arrow_forward
- Avogadro's Law Boyle's Law Charles' Law Guy-Lussac's Law TT The volume of a gas increases as the amount of gas increases. The volume of a gas increases as the pressure decreases. X X The pressure of a gas increases as X the temperature increases. I The volume of gas increases as the X temperature increases.arrow_forwardA 1.20 L weather balloon on the ground has a temperature of 25.0°C and is at atmospheric pressure (1.00 atm). When it rises to an elevation where the pressure is 0.740 atm, then the new volume is 1.80 L. What is the temperature (in °C) of the air at this elevation?arrow_forwardA 1.20 L weather balloon on the ground has a temperature of 25.0°C and is at atmospheric pressure (1.00 atm). When it rises to an elevation where the pressure is 0.730 atm, then the new volume is 1.80 L. What is the temperature (in °C) of the air at this elevation?arrow_forward
- 43arrow_forwardA sample contains 10.5 g N2 (MW = 28.02 g/mol), 6.5 g He (MW = 4.00 g/mol), and 34.6 g CO2 (MW = 44.01 g/mol). If this sample has a total pressure of 15.0 atm, calculate the partial pressure due to the He (PHe)Select one:12.3 atm 8.7 atm 5.0 atm 3.5 atmarrow_forward1. A cylinder contains 3.00 mol of Ar, 6.00 mol of Ne, and 1.00 mol of He. At 200 K, the total pressure is found to be 3.00 at What is the partial pressure of Ne gas in the cylinder? 01.80 atm 00.900 atm O2.00 atm 01.00 atm 18.0 atmarrow_forward
- A 10.0 L sample of carbon dioxide gas has a temperature of 500.0 K. If the volume of the gas is decreased to 2.2 L at constant pressure, what is the final temperature of the gas? 2300 K 110 K 0.044 K 23 Karrow_forwardA gas-filled weather balloon has a volume of 52.0 L at ground level, where the pressure is 761 mmHg and the temperature is 23.2 °C. After being released, the balloon rises to an altitude where the temperature is -8.76 °C and the pressure is 0.0830 atm. What is the weather balloon's volume at the higher altitude? V= Larrow_forwardA 7.94-g piece of solid CO, (dry ice) is allowed to sublime in a balloon. The final volume of the balloon is 1.00 L at 301 K. What is the pressure of the gas? 4.46 atm O 3.11 atm 0.224 atm O 1.96 x 10² atmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY