
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
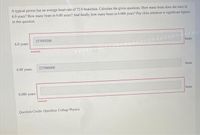
Transcribed Image Text:A typical person has an average heart rate of 72.0 beats/min. Calculate the given questions. How many beats does she have in
6.0 years? How many beats in 6.00 years? And finally, how many beats in 6.000 years? Pay close attention to significant figures
in this question.
beats
227059200
6.0 years:
Incorrect
beats
227000000
6.00 years:
beats
6.000 years:
Incorrect
Question Credit: OpenStax College Physics
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Recently one month's production of sodium hydroxide in the US was 2.29 billion pounds. The density of sodium hydroxide is 2.130 g/cm³. What is the volume in cubic kilometers that were produced?Recently one month's production of sodium hydroxide in the US was 2.29 billion pounds. The density of sodium hydroxide is 2.130 g/cm³. What is the volume in cubic kilometers that were produced?Carl is approaching the Canadian border on a trip from Vermont to Montreal, Canada, and the gas in his tank is low. He wants to determine if it would be more cost efficient to buy gas before or after crossing the border. He consults Gas Buddy and finds that the cost of gas in the US is 2.45 USD per gallon, while just across the border it is $1.40 CAD per liter. The currency exchange rate is $0.750 USD per $1.000 CAD. How much more gas (in gallons) will he get for $20 USD if he buys the gas before crossing the border instead of buying it in Canada? (1.000 liter = 0.264 US gallons).arrow_forwardLead metal can be extracted from a mineral called galena, which contains 86.6% lead by mass. A particular ore contains 68.5% galena by mass. If the lead can be extracted with 92.5% efficiency, what mass of ore is required to make a lead sphere with a 3.00 cm radius?arrow_forwardA cubic object has a side length of 3.17 cm. It is found to be a pure substance with a mass of 41.18 grams. What is the density of the substance in g/cm3? Numbers only, answer to 2 decimal places. Volume of cube = (Side)3arrow_forward
- Celsius and Fahrenheit Temperature Conversion Conver 100.0°F to degrees Celsius and to kelvins. Convert 2,200°F to degrees Celsius and to kelvins. An individual who is ill has a Temperature of 40.0°C. Normal body temperature is 37.0°C. This represent in °F? What is the individual's body temperature in °F?arrow_forwardA 76.00 pound flask of mercury costs $162.00. The density of mercury is 13.534 g/cm³. Find the price of one cubic inch of mercury by calculating intermediate values. What is the price of one pound of mercury? $ 2.131 What is the price of 1 g of mercury? $ What is the price of 1 cm³ of mercury? S What is the price of 1 in³ of mercury?arrow_forwardConsider a simple economy that produces only air fryers. The following table contains information on the economy's money supply, velocity of money, price level, and output. For example, in 2021, the money supply was $400, the price of a air fryer was $12.50, and the economy produced 800 air fryers. Fill in the missing values in the following table, selecting the answers closest to the values you calculate. Quantity of Money Price Level Year (Dollars) Velocity of Money (Dollars) 2021 400 12.50 2022 416 25 Quantity of Output (Air fryers) 800 800 Nominal GDP (Dollars) 10,000.00 from 2021 to 2022. Since air fryer output did not change from 2021 to 2022 and the velocity of the change in the money supply was reflected in changes in the price level. The inflation rate from The money supply grew at a rate of 4% money increased 2021 to 2022 wasarrow_forward
- A 76.00 pound flask of mercury costs $150.00. The density of mercury is 13.534 g/cm³. Find the price of one cubic inch of mercury by calculating intermediate values. What is the price of one pound of mercury? What is the price of 1 g of mercury? What is the price of 1 cm of mercury? What is the price of 1 in' of mercury?arrow_forwardFrom your home to CNM is 1,787 steps, it takes you 13.4 minutes to do this walk, If you take 1,832 steps each mile, how many miles per hour are you walking? Give answer in standard notation with correct significant figuresarrow_forwardAdd or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 5.97 g 0.5 g g 9.800 g + 0.9 g g 4.87 g 0.570 g %3Darrow_forward
- A 76.00 pound flask of mercury costs $148.50. The density of mercury is 13.534 g/cm3. Find the price of the cubic mercury by calculating intermediate values. What is the price of one pound of mercury? What is the price of 1 g of mercury? What is the price of 1cm3 of mercury? What is the price of 1 in3 of mercury?arrow_forwardan inflatable pool toy is filled with 8.82 L of air in the summer when the temperature is 29.81. the toy is left outside until winter. what is the volume of air (in L) in the toy when the temperature drops to -12.65. provide your answer with two decimalsarrow_forwardComplete the following table. Be sure each of your answer entries has the correct number of significant digits. food energy content when eaten cal kcal kJ a 12-ounce soft drink ×1.50105 a raw carrot 30.0 a slice of cooked bacon 151.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY