
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
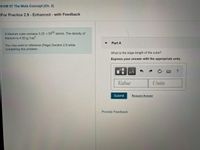
Transcribed Image Text:<HW 07 The Mole Concept (Ch. 2)
For Practice 2.9 - Enhanced - with Feedback
A titanium cube contains 3.25 x1025 atoms. The density of
titanium is 4.50 g/cm
Part A
You may want to reference (Page) Section 2.9 while
completing this problem.
What is the edge length of the cube?
Express your answer with the appropriate units.
HA
Value
Units
Submit
Request Answer
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many atoms are there in each of the following substances? 1.88 g Bi Express your answer using three significant figures. 8.0x10-2 g Sr Express your answer using two significant figures. 5.18 g P Express your answer using three significant figures. 2.36 g Hg Express your answer using three significant figures.arrow_forwardWhich one of the following would have approximately the same mass as 40.0 cm³ of lead (d = 11.3 g/cm³)?arrow_forwardPlease answer question number 2 correctly with the correct answer. there is a picture of an example of a ''Dimensional analysis Format' below. please write out all work showing the math in dimensional analysis format. (please show all work) Question 8: Calculate the mass in grams of each of the following: C.) 0.00100 mol silicon tetrahydride (SiH4) D. ) 1.50 x 10^-2 mol molecular oxygen (O2) E. 2.30 mol ethylene glycol (C2H6O2)arrow_forward
- A 81.15 g sample of a compound containing potassium, chromium and oxygen is analyzed as containing 21.57 g potassium and 28.69 g of chromium.. What mass of oxygen is contained in 287.1 g of this compound? Express your answer in decimal notation rounded to the correct number of significant figures.arrow_forward248024132 References Use the References to access important values if needed for this question. Under certain conditions, the substances sulfur dioxide and oxygen combine to form sulfur trioxide. If 28.8 grams of sulfur dioxide and 7.2 grams of oxygen combine to form sulfur trioxide, how many grams of sulfur trioxide must form? Mass= Submit Answer tion of Mass: Macroscopic: 1.arrow_forwardPART 1 Prelab Question = tr²l_ (I=length of wire) The density of copper at 20°C is 0.896 kg/dL at 20°C. A copper wire has a diameter of 0.110 mm. If the copper wire consists of 1.61376 x 1021 atoms of copper, calculate the length of the copper wire.arrow_forward
- Mass of one a mu)Carbon Type 1 12.0Carbon Type 2 13.0 What is the average mass of carbon in this sample? Is this number on the periodic table?( it may be rounded off ) where?arrow_forwardAn automobile gasoline tank holds 21 kg of gasoline. When the gasoline burns, 85 kg of oxygen is consumed, and carbon dioxide and water are produced. What is the total combined mass of carbon dioxide and water that is produced? Express your answer with the appropriate units.arrow_forwardCalculate the mass of hydrazine (N_2H_4) that contains a billion (1.000 x 10^9) nitrogen atoms. Be sure your answer has a unit symbol if necessary, and round it to significant digits. NOTE: underscore represents the subscriptarrow_forward
- Atoms are the smallest spherical particles that make up any matter. If 6.0x1023 atoms of aluminum weigh 27 g, calculate mass of 1 atom of aluminum in picograms (pg).arrow_forwardCalculate the number of hydrogen atoms in a 130.0 g sample of ammonia (NH₂). ES Be sure your answer has a unit symbol if necessary, and round it to 4 significant digits. f 0 D O x10 X Garrow_forwardAn aluminum atom has a mass of 4.48 x 10 - 23 g and a small airplane has a mass of 5000. kg. Use this information to answer the question below. Be sure your answers have the correct number of significant digits. What is the mass of 1 mole of aluminum atoms? x10 Round your answer to 3 significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY