
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:A thermometer is taken from a room where the temperature is 18°C to the outdoors, where the
temperature is -12°C. After one minute the thermometer reads 9°C.
(a) What will the reading on the thermometer be after 3 more minutes?
-1.7
(b) When will the thermometer read -11°C?
minutes after it was taken to the outdoors.
Expert Solution
arrow_forward
Step 1: Given details
The rate at which a body’s temperature changes is directly proportional to the temperature difference between the body and its surroundings.
The formula for the Newton's law of cooling is expressed as
Here,T(t) is the temperature of the object at a certain time t
Ts is the temperature of the surroundings. To is the initial temperature and is the cooling constant.
Provided, temperature of the surroundings(Ts) is -12oC and initial temperature is 18oC(To)
After one minute the thermometer reads 9 degrees C. i.e.
Step by stepSolved in 4 steps with 14 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
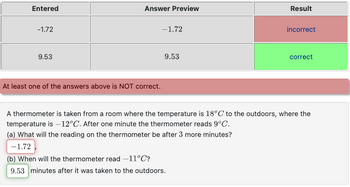
Transcribed Image Text:Entered
-1.72
9.53
Answer Preview
At least one of the answers above is NOT correct.
-1.72
9.53
Result
(b) When will the thermometer read -11°C?
9.53 minutes after it was taken to the outdoors.
incorrect
correct
A thermometer is taken from a room where the temperature is 18°C to the outdoors, where the
temperature is -12°C. After one minute the thermometer reads 9°C.
(a) What will the reading on the thermometer be after 3 more minutes?
-1.72
Solution
by Bartleby Expert
Follow-up Question
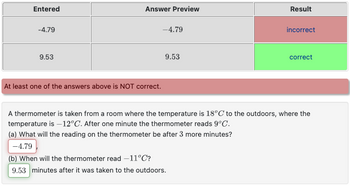
Transcribed Image Text:Entered
-4.79
9.53
Answer Preview
At least one of the answers above is NOT correct.
-4.79
9.53
Result
incorrect
correct
A thermometer is taken from a room where the temperature is 18°C to the outdoors, where the
temperature is -12°C. After one minute the thermometer reads 9°C.
(a) What will the reading on the thermometer be after 3 more minutes?
-4.79
(b) When will the thermometer read -11°C?
9.53 minutes after it was taken to the outdoors.
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question

Transcribed Image Text:Entered
-1.72
9.53
Answer Preview
At least one of the answers above is NOT correct.
-1.72
9.53
Result
(b) When will the thermometer read -11°C?
9.53 minutes after it was taken to the outdoors.
incorrect
correct
A thermometer is taken from a room where the temperature is 18°C to the outdoors, where the
temperature is -12°C. After one minute the thermometer reads 9°C.
(a) What will the reading on the thermometer be after 3 more minutes?
-1.72
Solution
by Bartleby Expert
Follow-up Question

Transcribed Image Text:Entered
-4.79
9.53
Answer Preview
At least one of the answers above is NOT correct.
-4.79
9.53
Result
incorrect
correct
A thermometer is taken from a room where the temperature is 18°C to the outdoors, where the
temperature is -12°C. After one minute the thermometer reads 9°C.
(a) What will the reading on the thermometer be after 3 more minutes?
-4.79
(b) When will the thermometer read -11°C?
9.53 minutes after it was taken to the outdoors.
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When 3 kg of water vapor cools to a temperature of 373 k, the value of q=-1950 kJ. If the specific hear of water vapor is 1.86 J/g. degrees Celsius, what is the initial temperature of the water vapor in degrees celsius? Please answer with a clear explanation and steps!arrow_forwardWashington, D.C. has an average January temperature of 34.9 degrees Fahrenheit. What is this average if temp is expressed in Celsius? (Round to two decimal places.) [Hint: Conversion between Fahrenheit and Celsius is a linear transformation: Fahrenheit = Celsius*1.80 + 32, or Celsius = Fahrenheit*(1/1.80) - (32/1.80)]arrow_forwardHow many significant figures are there in each of the fol- lowing quantities? (a) Distance from New York City to Wellington, New Zealand, 14,397 km 1 (b) Average body temperature of a crocodile, 25.6 °C (c) Melting point of gold, 1064 °C (d) Diameter of an influenza virus, 0.000 01 mm (e) Radius of a phosphorus atom, 0.110 nmarrow_forward
- 1.a What temperature in Kelvin is -145°C (b)What temp in Celsius is 91 °F? (c) Which of the following is not an equivalent combined unit of m/s2 ? Group of answer choices km/hr2 kg/m2 m2/ms2 cm/ms2arrow_forwardQ13.arrow_forwardIf 2.346 and 12.97 are two significant figures, use the rules for significant figures to calculate the following: (a)2.346 + 12.97 = (c) 2.346 * 12.97 = (b)2.346 - 12.97 = (d) 2.346 / 12.97 =arrow_forward
- 1.111 In the hospital, your doctor orders 100. mg of medication per hour. The label on the IV bag reads 5.0 g/1000 mL The IV administration set delivers 15. gtts/mL, where the unit gtts denotes drops of liquid as explained in Problem 1.51. (a) How many mL should infuse each hour? (b) The current drip rate is set to 10. gtts/min. Is this correct? If not, what is the correct drip rate?arrow_forwardA pediatric patient with a body mass of 75.0 lb is prescribed Propranolol for arrhythmia. The recommended dose is 3.0 mg per kg per day, given in divided doses every 6.00 hours. How many mg of Propranolol should be given for each dose? With the correct amount of Sig Figs.arrow_forwardA sheet of gold weighing 9,6 g and at a temperature of 16.4 °C is placed flat on a sheet of iron weighing 18.5 g and at a temperature of 50.1 "C. What is the final temperature of the combined metals? Assume that no heat is lost to the surroundings Be sure your answer has the correct number of significant digits. 0arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY