
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
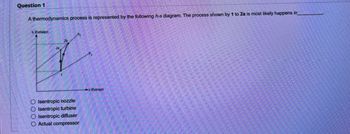
Transcribed Image Text:Question 1
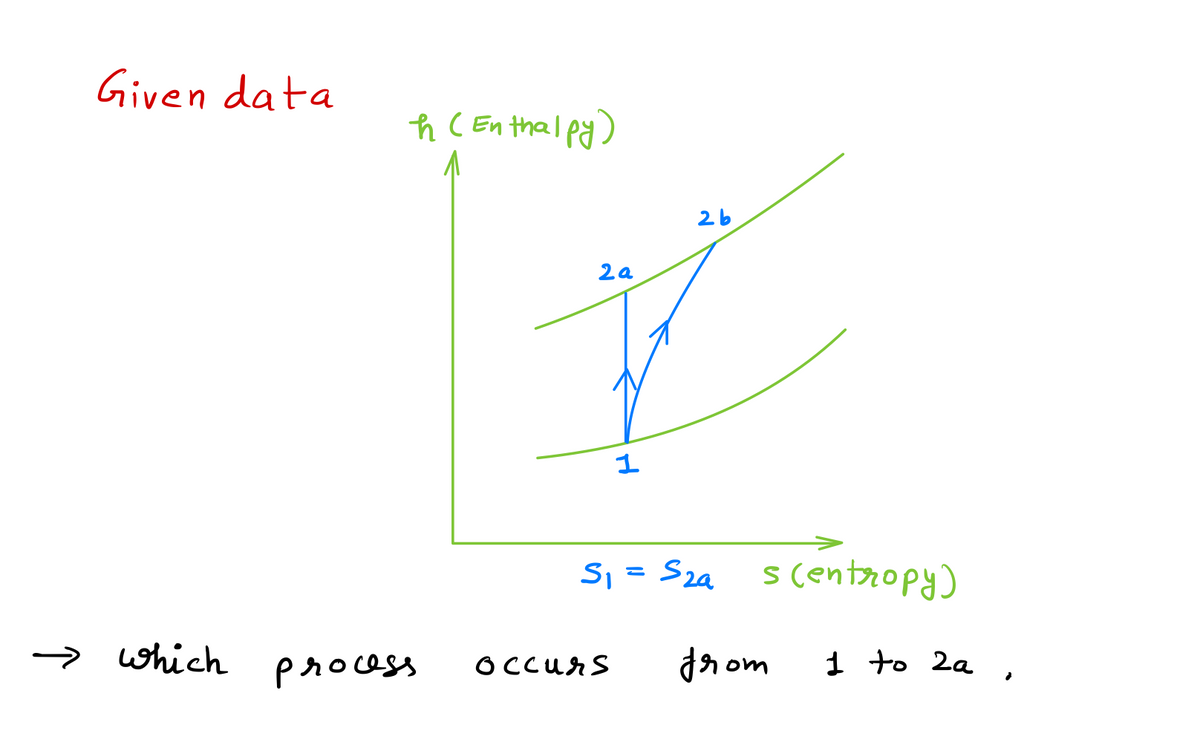
A thermodynamics process is represented by the following h-s diagram. The process shown by 1 to 2a is most likely happens in
Enthalpy
O Isentropic nozzle
O Isentropic turbine
O Isentropic diffuser
O Actual compressor
Entropy)
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- If you have a material that is initially hard and strong, would you expect it to cyclically harden or soften? What would be a way of characterizing how strong it must be initially to make your answer a bit more quantitative?arrow_forwardA 2.00 mol sample of an ideal diatomic gas is taken through a reversible cycle A → B → C → A as shown in the figure below. Process A → B is isothermal expansion. For the cycle, calculate: a) the work done by the gas, b) the heat energy added to the gas, c) the heat energy exhausted by the gas, d) the thermal efficiency, e) the change in entropy of the gas. f) Determine the efficiency of a Carnot engine operating between the same temperature extremes, R = 8.314 J/mol K Data at vertices for the above PV diagram are as follow: PA = 5.00 x 105 Pa, VA = 1.00 x 10 -2 m3, PB = 1.25 x 105 Pa, VB = 4.00 x 10 -2 m3 PC = 1.25 x 105 Pa, VC = 1.00 x 10 -2 m3arrow_forwardPlease solve all parts solution nee...darrow_forward
- Argon gas flows through a well-insulated nozzle at steady state. The temperature and velocity at the inlet are 550°R and 150 ft/s, respectively. At the exit, the temperature is 460°R and the pressure is 40 Ibf/in?. The area of the exit is 0.0085 ft?. Use the ideal gas model with k = 1.67, and neglect potential energy effects. Determine the velocity at the exit, in ft/s, and the mass flow rate, in Ib/s.arrow_forwardExample 7: Throttling Water is throttled from 1 bar, 60 °C, to 0.5 bar, and to 0.1 bar. In each case calculate the exit temperature and the entropy generation. ab k 11°C Mostly cloudy Esc FnLock 1 1 2 Q FI A Z @ 2 W S # 3 X Saluting ‒‒ - E D Q Search F4 $ 4 C R F V 6 G V B & 7 H FB U N * 8 J F9 1 ( 9 F10 K M O F11 O P JE 3 ; + ( O [ 4x 11 ? 1 9:49 AM 11/1/2023 Delete A Backspace 0 Enarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY