
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
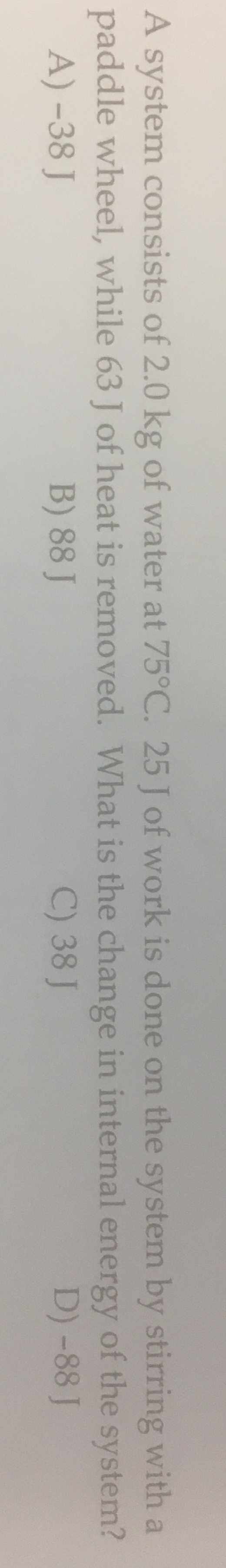
Transcribed Image Text:A system consists of 2.0 kg of water at 75°C. 25 J of work is done on the system by stirring with a
paddle wheel, while 63 J of heat is removed. What is the change in internal energy of the system?
A) -38 J
B) 88 J
C) 38 J
D) -88 J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 3 images

Knowledge Booster
Similar questions
- A heat engine under development is expected to transfer heat from reservoir A to reservoir B. Which is a requirement for the two reservoirs? A) The temperature of reservoir A must be greater than the temperature of reservoir B. B) The temperature of reservoir A must be equal to the temperature of reservoir B. C) The internal energy of reservoir A must be greater than the internal energy of reservoir B. D) internal energy of reservoir A must equal the internal energy of reservoir B.arrow_forward6. Consider an isothermal process. If the heat flows 10.0 J from the system, find the change of internal energy. A.−10.0 J。 B.10.0 J。 C.5.00 J。 D.0.00 J。arrow_forward400 J of work are done on a system in a process that decreases the system's thermal energy by 300 J. You may want to review (Pages 524 - 525). How much heat energy is transferred? Express your answer with the appropriate units. u Value Submit % O O A Units www. ? X Previous Answers Request Answerarrow_forward
- 3600 J of work are done on a container of Argon gas compressing it down from its original state of 1.2 atm, 0.04 m , 19°C. If the gas warms to 110°C how much heat in Joules must enter or leave the system? Heat entering is +, heat leaving is-. Q = %3Darrow_forwardA certain gas is compressed adiabatically. The amount of work done on the gas is 800 J. What is the change in the internal energy of the gas? Question 4 options: 400 J More information is needed to answer this question. 0 J -800 J 800 J For vapor water at 2°C Question 5 options: Cp < Cv. Cp > Cv. Cp = Cv. More information is needed to answer this question.arrow_forwardWhich of the following can effectively lower the internal energy of a room? A A fan B A refrigerator with its door left wide open C An air - conditioner partially exposed to the atmosphere D A refrigerator with its door closed 9.arrow_forward
- During the adiabatic expansion, the temperature of 0.1 mol of oxygen drops from 30C to 10C. a) How much work does the gas do? b) How much heat is added?arrow_forwardIf a system has a change in energy of 400 J and produces 200 J of work, how much heat was added to the system? 89°F Very high UV Q 800 J 200 J -200 J 600 J F2 2 W S F3 Q+ 3 E F4 D $ 4 F5 R F % 5 F6 O Search T 77 G F7 Y Y F8 & 87 H U F9 * 00 8 J G F10 LA 9 O F11 K F12 Prt Sc Insert no ha Del Backspacearrow_forward3. A system does 1.80×10° Jof work while 7.50×108 J of heat transfer occurs to the environment. What is the change in internal energy of the system assuming no other changes (such as in temperature or by the addition of fuel)?arrow_forward
- Over the course of a day, 4.0 kg of water evaporates from the leaves of a corn plant. a) How much energy is required to evaporate the water? (Assume that the temperature of the leaves is 30C.) b) If the plant is active for 12 hours, how much power does this correspond to? You can think of this as the necessary power to drive transport in the plant.arrow_forward800 J of work are done BY a system in a process that decreases the thermal energy of the system by 50 J. How much heat is transferred TO the system during this process? If the system gains heat, answer with a positive number. If the system loses heat, answer with a negative number. (in J) -750 750 -850 -50 0arrow_forwardAn electric power plant uses energy from burning coal to generate steam at 450oC. The plant is cooled by 20oC water from a nearby river. If burning coal provides 100MJ of heat, what is the theoretical minimum amount of heat that must be transferred to the river during the conversion of heat to electric energy? a- 100MJ B- 90MJ c- 60MJ D- 40MJarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON