
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Answer these
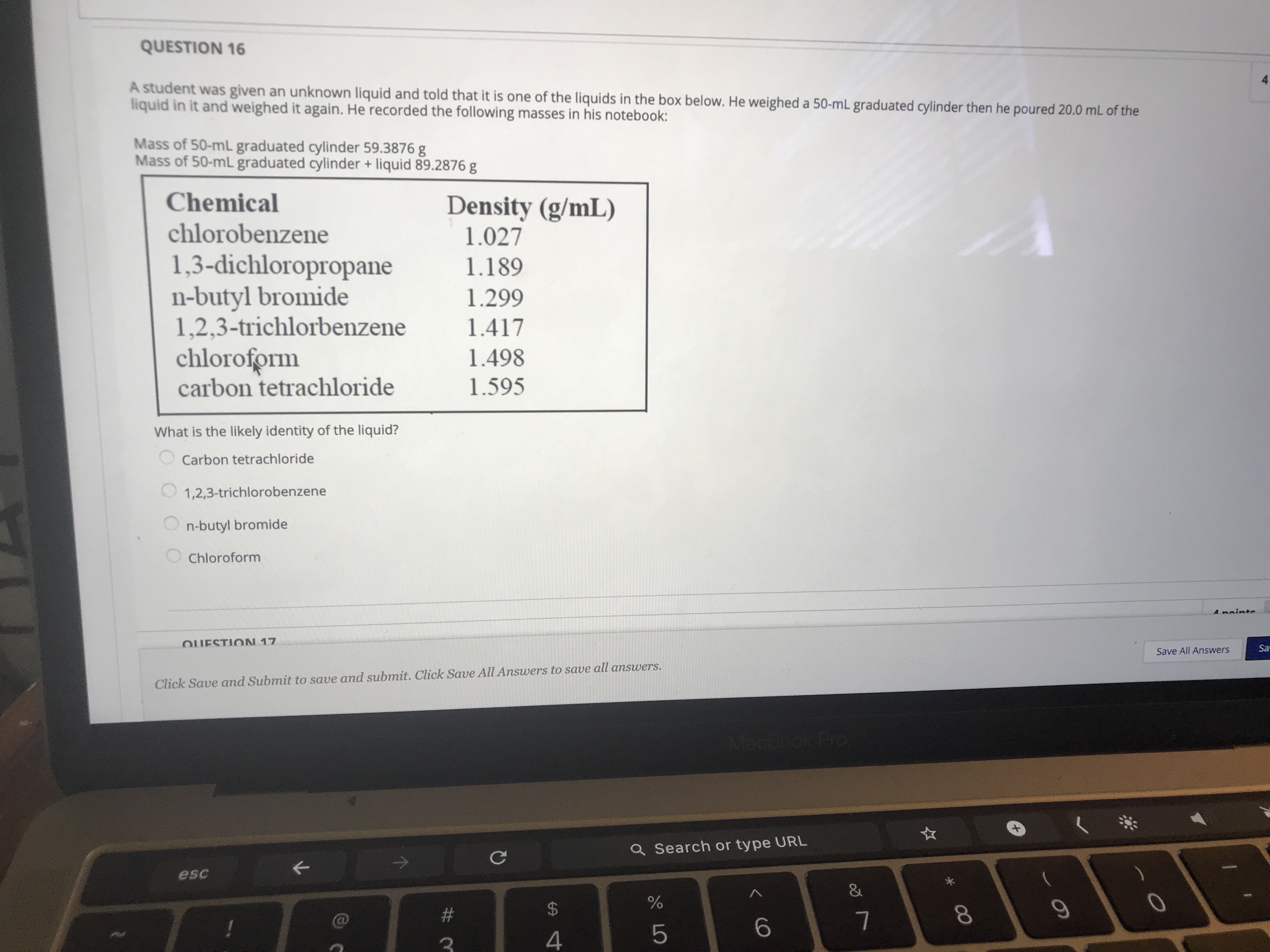
Transcribed Image Text:A student was given an unknown liquid and told that it is one of the liquids in the box below. He weighed a 50-mL graduated cylinder then he poured 20.0 mL of the
liquid in it and weighed it again. He recorded the following masses in his notebook:
Mass of 50-mL graduated cylinder 59.3876 g
Mass of 50-ml graduated cylinder + liquid 89.2876 g
Chemical
Density (g/mL)
1.027
chlorobenzene
1,3-dichloropropane
n-butyl bromide
1,2,3-trichlorbenzene
chloroform
carbon tetrachloride
1.189
1.299
1.417
1.498
1.595
What is the likely identity of the liquid?
Carbon tetrachloride
1,2,3-trichlorobenzene
n-butyl bromide
Chloroform
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the frequency of visible light having a wave length of 486 nm. Answer in s-1 to 3 sig figsarrow_forwardGiven f(x) = x- 7, what will f(12) be equal to? 12 19arrow_forwardNow draw two waves on your own sheet of paper. Predict what would happen if they arrive at the same spot at the same time (also known as "in-phase"). What would be the effect? Enter your answer here Save Answerarrow_forward
- 337.1 nm(wavelength of a nitrogen laser) Express your answer using three significant figures.arrow_forward5:34 PM Fri Oct 8 AA www-awn.aleks.com G periodic table -.. Favorites - YouT... TV fuboTV - Watch... ALEKS - Caliyah... G kno3 acid or ba... G linear program... G write formulas f... Solved A O CHEMICAL REACTIONS Predicting whether simple electrochemical reactions happen Caliyah v Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols. chemical situation chemical equation reaction? A strip of solid copper metal is put into a beaker of 0.068M SnCl, solution. O yes O no 13 A strip of solid tin metal is O yes put into a beaker of O no 0.037M Cu(NO3)2 solution.arrow_forwardMake sure corrarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY