
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
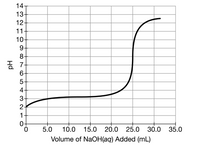
A student titrated 50.0mL of a 0.10M solution of a certain weak acid with NaOH(aq). The results are given in the graph above.
What is a pH value between 2.5- 7.5 where the concentration of the weak acid being titrated is less than the concentration of its conjugate base?
Transcribed Image Text:14-
13-
12-
11-
10-
9-
8-
5-
3-
2-
1
5.0
10.0
15.0
20.0
25.0
30.0 35.0
Volume of NaOH(aq) Added (mL)
N O 5 ¢32
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student used a coffee-cup calorimeter to determine the enthalpy of solution for NH4NO3.When NH4NO3 is added to water, there is a decrease in temperature of the solution. Is the solution process exothermic or endothermic? (a) endothermic (b) exothermicarrow_forwardOne way to lose weight is to exercise! Walking briskly at 4.0 miles per hour for an hour consumes about 400 kcal of energy. How many hours would you have to walk at 4.0 miles per hour to lose one pound of body fat? One gram of body fat is equivalent to 7.7 kcal of energy. There are 454 g in 1 lb.arrow_forwardA rebreathing gas mask contains potassium superoxide, KO2, which reacts with moisture in the breath to give oxygen. 4KO2(s)+2H2O(l)4KOH(s)+3O2(g) Estimate the grams of potassium superoxide required to supply a persons oxygen needs for one hour. Assume a person requires 1.00 102 kcal of energy for this time period. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 1.00 102 kcal of heat, calculate the amount of oxygen consumed and hence the amount of KO2 required. The ff0 for glucose(s) is 1273 kJ/mol.arrow_forward
- The carbon dioxide exhaled in the breath of astronauts is often removed from the spacecraft by reaction with lithium hydroxide 2LiOH(s)+CO2(g)Li2CO3(s)+H2O(l) Estimate the grams of lithium hydroxide required per astronaut per day. Assume that each astronaut requires 2.50 103 kcal of energy per day. Further assume that this energy can be equated to the heat of combustion of a quantity of glucose, C6H12O6, to CO2(g) and H2O(l). From the amount of glucose required to give 2.50 103 kcal of heat, calculate the amount of CO2 produced and hence the amount of LiOH required. The H for glucose(s) is 1273 kJ/mol.arrow_forward87. What volume of 0.151 N NaOH is required to neutralize 24.2 mL of 0.125 N H2SO4? What volume of 0.151 N NaOH is required to neutralize 24.2 n1L of 0.125 M H2SO4?arrow_forwardAmoxicillin is an antibiotic packaged as a powder. When it is used to treat babies and small animals, the pharmacist or veterinarian must suspend it in water, so that it can be administered orally with a medicine dropper. The label says to dispose of unused suspension after 14 days. It also points out that refrigeration is required. In the context of this chapter, what is implied in the latter two statements?arrow_forward
- Define the joule in terms of SI base units.arrow_forward4-77 To convert 1 mol of iron(III) oxide to its elements requires 196.5 kcal: How many grams of iron can be produced if 156.0 kcal of heat is absorbed by a large-enough sample of iron(III) oxide?arrow_forwardAs part of the aspirin synthesis lab the orgo students also had to perform the following calculation to demonstrate their knowledge. Are you able to help them work this out? Saponification is a process in which soap is produced from the chemical reaction between animal fat (triglycerides) and a strong base such as KOH. An example of such balanced chemical reaction is shown here: C51H98O6 + 3KOH → C3H5(OH)3 + 3C16H31O2K (Triglyceride) (Soap) if during the saponification reaction 231.5 g of C51H98O6 is mixed with 231.5 g of KOH and 160 g of soap is produced. Calculate the theoretical yield of soap C16H31O2K and indicate who is the limiting reactant. (Provide your answer to 2 decimal places) Calculate the percent yield for this reaction (Provide your answer to 1 decimal place) *Show ALL steps and mathematical equations involved in your calculations to help the orgo students. Remember to label all…arrow_forward
- For the reaction 2N2O5(g) ® 4NO2(g) + O2(g), the following data were collected. t (minutes) [N2O5] (mol/L) 0 1.24 x 10–2 10 0.92 x 10–2 20 0.68 x10–2 30 0.50 x 10–2 40 0.37 x 10–2 50 0.28 ´ 10–2 70 0.15 ´ 10–2 The initial rate of production of NO2 for this reaction is approximately: A) 6.4 x10–4 mol/L • min B) 3.2 x10–4 mol/L • min C) 1.24 x 10–2 mol/L • min D) 1.6 x 10–4 mol/L • min E) none of thesearrow_forwardIn this experiment you will measure the enthalpy of three processes. Reaction 1 NaOH (s) → NaOH (aq) AH1 Reaction 2 NaOH (aq) + HCI (aq) → H2O (I) + NaCI (aq) AH2 Reaction 3 NaOH (s) + HCI (aq) → H2O (I) + NaCI (aq) AH3arrow_forwardreaction Mg(s) + Cl₂(g) → MgCl₂ (s) CH₂(g) + 20₂ (g) K Br(aq) + AgNO₂ (aq) KNO₂ (aq) + Ag Br(s) HBr(aq) + NaOH(aq) → NaBr(aq) + H,O (1) CO₂(g) + 2H₂O(g) → K 10 0 0 0 0 ОС 00000000 type of reaction (check all that apply) combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition X precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base Ś ? 0 000 000arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning