
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
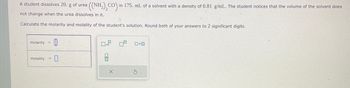
Transcribed Image Text:A student dissolves 20. g of urea
not change when the urea dissolves in it.
Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits.
molarity
molality=
((NH₂)₂CO) in 175. mL of a solvent with a density of 0.81 g/mL. The student notices that the volume of the solvent does
00
0x0
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The solubility of solid X is given below. What is the maximum percent recovery if a student wanted to purify 4.20 g of crude solid X from 75 mL of water? Provide explanations with your calculations Solubility (in 100 mL of water) Temperature 5.5 g 100 C 0.53 g 0 Carrow_forwardSubject--chemistryarrow_forwardA student dissolves 18. g of biphenyl (C₁2H₁0) in 100. mL of a solvent with a density of 1.04 g/mL. The student notices that the volume of the solvent does not change when the biphenyl dissolves in it. Calculate the molarity and molality of the student's solution. Be sure each of your answer entries has the correct number of significant digits. molarity 1 = molality 0 = x10 0|0arrow_forward
- Determine the percent by mass of MgCl2 if 3.1 g of MgCl2 is dissolved in 41.1 g of water.arrow_forwardAt 70 °C, the solubility of an unknown solute is 65.7 g/100.0 g of water. What mass of the solute can dissolve in 125.5 g of water at the same temperature?arrow_forwardCalculating molality.arrow_forward
- A solution containing 25.0 g of protein per liter has an osmotic pressure of 8.3 mmHg at 37°C. What is the molar mass of the protein? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b с d e 5.8 X 104 g mol-1 7.3 X 104 g mol-1 9.3X 104 g mol-1 1.2 X 105 g mol-1 3.2 X 105 g mol-1arrow_forwardThe solubility of heptanol, CH3(CH₂)6OH, is 0.16 g per 0.10 L. What is the molarity of the solution? 0.073 0.013 0.0073 0.133 0.16arrow_forwardAn aqueous solution of ethanol (molar mass = 46.07 g/mol) has a molality of 12.15 m and a density of 1.100 g/mL. What is the molarity of ethanol in the solution?arrow_forward
- Use the solubility curve below to answer the following questions: 150 140 130 120 110 100 NANO3 90 80 70 60 NH&Cl KCI NaCi 50 40 30 20 KCIO3 10 Ce2(SOals O 10 20 30 40 50 60 70 80 90 100 Temperature (c) Q1) As the temperature increases, the solubility of the NH3 in water Q2) The solute, NH3, is MOST likely a (increases/decreases/stays the same). (solid, liquid, or gas). Q3) How many grams of NH4CI are dissolved in 500 g of water at 90 °C? Q4) If you had 50 grams of NH4CI in 100 g of water at 40 °C, would the solution be saturated, unsaturated, or supersaturated? Q5) You heat a solution of KCIO3 to 80°C and saturate the solution. You then allow the solution to cool down to 50°C. How many grams of solute would precipitate out of the solution? NH3 per 100 g H,0 Grams of solute ONO CONarrow_forwardDetermine the freezing point (in °C) of a solution that contains 204 g of C6H1206 dissolved in 234 mL of acetic acid (density = 1.05 g/mL). Pure acetic acid has a freezing point of 16.6°C and a K = 3.90°C/m.arrow_forwardConcentration is the amount of solute dissolved in a certain amount of solution: Solution concentrations are usually given as one of the following: ● Mass percent (m/m)-the mass of the solute in grams for exactly 100 g of solution • Volume percent (v/v)-the volume of solute in exactly 100 mL of solution • Mass/volume percent (m/v)-the mass of solute in grams for exactly 100 mL of solution ● Molarity (M)-the number of moles of solute in exactly 1 L of solution Part A Calculate the mass percent (m/m) of a solution prepared by dissolving 43.37 g of NaCl in 171.3 g of H₂O. Express your answer to four significant figures. ► View Available Hint(s) Mass percent (m/m) = concentration of a solution = _amount of solute amount of solution Submit Π| ΑΣΦ ? % P Pearson Copyright © 2023 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us |arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY