
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:A steel cylinder containing 25 kg of CCl2 F2 in the form of
liquid and vapor is set outdoors on a warm day (25 °C).
What is the approximate pressure of the vapor in the
cylinder?
Pressure =
atm
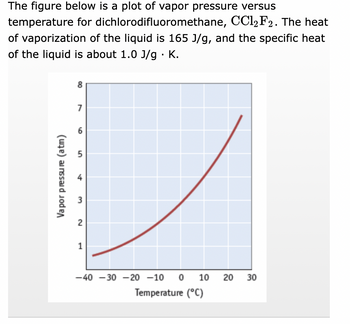
Transcribed Image Text:The figure below is a plot of vapor pressure versus
dichlorodifluoromethane, CCl2 F2. The heat
temperature for
of vaporization of the liquid is 165 J/g, and the specific heat
of the liquid is about 1.0 J/g. K.
Vapor pressure (atm)
8
7
6
5
4
3
2
1
-40 -30 -20 -10 0 0 10 20 30
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A metal reacts with aqueous hydrochloric acid to producehydrogen. The hydrogen (H2) is collected over water at25°C under a total pressure of 0.9900 atm. The vaporpressure of water at this temperature is 0.0313 atm. Calculate the mass of hydrogen per liter of “wet” hydrogen above the water, assuming ideal gas behaviorarrow_forwardA 6.30 g sample of solid Na3PO4⋅7H2O was heated such that the water turned to steam and was driven off. Assuming ideal behavior, what volume would that steam occupy at 1.00 atm and 100.0 °C?arrow_forwardA 0.765-gram sample of impure potassium chlorate KClO3was decomposed by thermal decomposition into oxygen gas and potassium chloride. The oxygen, 222.50 mL was collected in a eudiometer over water at a temperature of 20.00 C and a pressure of 777 mm Hg. The water level in the eudiometer is 42.2 mm above the water level in the beaker. The vapor pressure of water at this temperature is 17.54 mm Hg Determine the purity of the original potassium chlorate samplearrow_forward
- Can water stay liquid below zero degrees Celsius?arrow_forwardAt standard temperature and pressure the molar volumes of CO2CO2 and NH3NH3 gases are 22.31 and 22.40 LL, respectively. The density of crystalline CO2CO2 at 160 KK is 1.56 g/cm3g/cm3. Calculate the molar volumes.arrow_forwardStudy the following phase diagram of Substance X. pressure (atm) 36 18 0. 0 solid liquid 200 gas 400 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at - 165. °C and 26.2 atm What will be the state of the sample? Suppose the temperature is held constant at -165. °C but the pressure is decreased by 18.3 atm. What will happen to the sample? ✓ (choose one) C solid liquid gas îarrow_forward
- A 250 mL flask contains air at 1.005 atm and 24.7 °C.5 mL of ethanol is added, the flask is immediately sealed and then warmed to 95.5 °C, during which time a small amount of the ethanol vaporizes. The final pressure in the flask (stabilized at 95.5 °C) is 2.876 atm. a) What is the partial pressure of air, in the flask at 95.5 °C? b)A solution which contains 77.5 g of an unknown molecular compound in 342 g of water freezes at -5.18°C.What is the molar mass of the unknown?arrow_forwardWhat would be a better solvent for sulfur dichloride, water or carbon tetrachloride?arrow_forwardHow many of the following forms a molecular solid? Ag • CO2 • RbI • Al203 • H20 Gold С10Н22 I2 3 5 4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY