Discuss the inductive and mesomeric (resonance) effects of the following compounds in order of acidity in descending order.


When any atom (X) is more electronegative than carbon, it attracts the bond pairs of electron and said to have -I effect that is negative inductive effect.
Similarly for mesomeric effect, when a π-system accepts the electron, it is said to have a -M effect that is negative mesomeric effect.
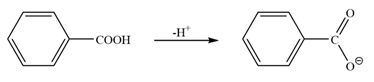
a) In the first molecule there is no any group attached to the benzene ring. Hence, there is neither stabilization nor destabilization of the conjugate base formed.
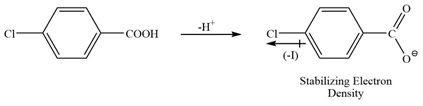
b) In the second structure there is a Cl group at para position. Cl group shows -I effect hence stabilizes the conjugate base but since it is at para position, the effect will be lower.
Step by step
Solved in 4 steps with 6 images









