
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:70
alll ll ?
..::0E is
اجابة قصيرة
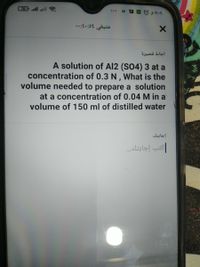
A solution of Al2 (S04) 3 at a
concentration of 0.3 N , What is the
volume needed to prepare a solution
at a concentration of 0.04 M in a
volume of 150 ml of distilled water
إجابتك
كتب إجابتك. .
is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student needs to prepare 50.0 mL of a 1.20 M aqueous H2O2 solution. Calculate the volume of 4.6 M H2O2 stock solution that should be used to prepare the solution.arrow_forwardWhat mass of K2CO3, in grams, is needed to prepare 211 mL of a solution having a potassium ion concentration of 0.147 M?arrow_forwardHelp asap! Be detailed plsarrow_forward
- A 15 mL Sample of 0.255 M cobalt(II) Chloride solution is diluted 500 mL. What is the new molarity of the diluted sample?arrow_forwardWhat mass of sodium hydroxide (NaOH, molar mass = 40.0 g∙mol–1) is needed to make 100.0 mL of a 0.125 M NaOH solution?arrow_forwardWhat mass of calcium chloride, CaCl2, in needed to prepare 2.657L of a 1.55 M solution?arrow_forward
- What is the molarity of a solution prepared by dissolving 26.2 g of NaNO3 in 500.0 mL of water?arrow_forwardVinegar is a solution that is 5% mass acetic acid (CH3COOH). What is the molarity of this solution (assume the density is 1.0 g/mL).arrow_forwarda) What mass of barium nitrate (Ba(NO3)2) would be required to prepare 2.000 L of a 0.0210 M aqueous solution of this salt? g(b) Calculate the mass percent of Ba(NO3)2 in this solution, assuming the density of the solution is 1.000 kg/L. %arrow_forward
- How many grams of aluminum chloride, AICI3, must be dissolved to prepare 250. mL of a 0.238 M aqueous solution of the salt? O7.93 grams 6.88 grams O7.64 grams O 3.57 grams hparrow_forwardcalculate the mass (g) of magnesium nitrate, Mg(NO3)2 required to prepare 2.00 L of 0.150 M Mg(NO3)2 solutionarrow_forwardIf 3.7 g of CuSO4 is dissolved in 5.0 x 101 g of H2O, what is the mass percent of CuSO4 in the solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY