
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
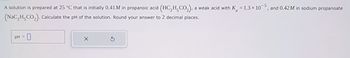
Transcribed Image Text:A solution is prepared at 25 °C that is initially 0.41M in propanoic acid (HC2H,CO₂), a weak acid with K = 1.3 × 105, and 0.42M in sodium propanoate
(NaC₂H CO₂). Calculate the pH of the solution. Round your answer to 2 decimal places.
pH = 0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The base protonation constant K of morpholine (C4H₂ONH) is 2.14 x 10 Calculate the pH of a 1.5 M solution of morpholine at 25 °C. Round your answer to 1 decimal place. pH = ☐ Xarrow_forwardThe base protonation constant K, of ammonia (NH,) is 1.8 × 10 °. Calculate the pH of a 0.86 M solution of ammonia at 25 °C. Round your answer to 1 decimal place. pH = |arrow_forwardA chemist dissolves 407. mg of pure hydrochloric acid in enough water to make up 230. mL of solution. Calculate the pH of the solution. Be sure your answer has the correct number of significant digits. x10arrow_forward
- A chemist dissolves 656. mg of pure barium hydroxide in enough water to make up 90. mL of solution. Calculate the pH of the solution. (The temperature of the solution is 25 °C.) Be sure your answer has the correct number of significant digits.arrow_forwardThe base protonation constant K₁ of lidocaine (C14H21 NONH) is 1.15 × 10 Calculate the pH of a 0.88 M solution of lidocaine at 25 °C. Round your answer to 1 decimal place. pH = ☐ xarrow_forwardHello can someone help me with the bottom problem i am confused and if anyone know a reagent list that would be helpful because online is all over the place.arrow_forward
- The acid dissociation constant K of carbonic acid (H,CO,) is 4.5 x 10. Calculate the pH of a 2.0M solution of carbonic acid. Round your answer to 1 decimal place. pH =arrow_forwardChemists have defined the pH (Hydrogen Potential) of a solution by pH = -log[H+] where [H+] represents the concentration of the hydrogen ion in moles per liter. The pH scale ranges from 0 to 14 depending on a solution’s acidity or alkalinity. Values below 7 indicate progressively greater acidity, while above 7 are progressively more alkaline. Normal, unpolluted rain has pH of about 5.6. Since the pH scale is logarithmic, keep in mind that there is tenfold in hydrogen ion concentration for each pH unit. An environmental concern involves the destructive effects of acid rainfall ever had a pH of 2.4, what is the hydrogen ion concentration? A. 0.004 mole per liter B. 0.002 mole per liter C. 0.39 mole per liter D. 0.25 mole per literarrow_forwardA solution is prepared at 25 °C that is initially 0.44M in ammonia (NH3), a weak base with Kô= 1.8 × 10¯ Calculate the pH of the solution. Round your answer to 2 decimal places. pH = 0 × S and 0.31M in ammonium bromide (NH4Br). Iarrow_forward
- (THANKS FOR TUTORING ME) Have you ever been swimming in a pool, and your eyes were red when you get out? This means the chemicals in the pool were not adjusted correctly! More specifically, the measurement of the pool's pH was not quite right. pH is a measurement of hydronium ions and can be modeled by the function f(x) = −log10x. The variable x represents the amount of hydronium ions, and f(x) gives the pH level. Different liquids have ideal pH values that are different. Water at 25 degrees Celsius has a pH of 7. Anything that has a pH less than 7 is called acidic, and a pH above 7 is basic, or alkaline. Seawater has a pH just more than 8, whereas lemonade has a pH of approximately 3. Create a graph of the pH function either by hand or using technology. Locate on your graph where the pH value is 0 and where it is 1. You may need to zoom in on your graph. Use your graph to find the ideal pH level if the amount of hydronium ions is raised to 0.50. The pool company developed new…arrow_forwardCalculate the pH of an aqueous solution that is ( Ka (HCN) = 6.20 × 10-10) a. 0.870 M in HCN. pH = b. 0.0870 M in HCN. pH = с. 8.70 х 10 М НCN. pH =arrow_forwardA chemist dissolves 369. mg of pure potassium hydroxide in enough water to make up 140. mL of solution. Calculate the pH of the solution. (The temperature of the solution is 25 °C.) Round your answer to 3 significant decimal places. ☐ x10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY