
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
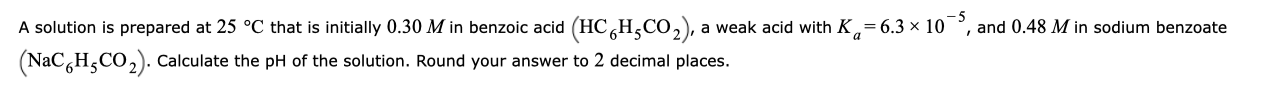
Transcribed Image Text:A solution is prepared at 25 °C that is initially 0.30 M in benzoic acid (HC,H,Co,), a weak acid with K,= 6.3 x 10
(NaC,H,CO,). Calculate the pH of the solution. Round your answer to 2 decimal places.
and 0.48 M in sodium benzoate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- A solution is prepared at 25 °C that is initially 0.086 M in propanoic acid (HC,H;CO,), a weak acid with K,= 1.3 x 10°, and 0.38 M in sodium propanoate Nac,H,CO,). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = | %3Darrow_forwardHow to calculate the pH of a buffer solution 1) Calculate the pH of a solution prepared by dissolving 1.90 g of sodium acetate, CH3COONa, in 67.5 mL of 0.10 Macetic acid, CH3COOH(aq). Assume the volume change upon dissolving the sodium acetate is negligible. K, of CH3COOH is 1.75 x 10-5. pH =arrow_forwardO ACIDS AND BASES Emily 1/5 Calculating the pH of a buffer A solution is prepared at 25 °C that is initially 0.069M in dimethylamine ((CH,), NH), a weak base with K= 5.4 x 10*, ', and 0.32M in dimethylammonium chloride ((CH3), NH,CI). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = Eplanation Check © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center 21 étvarrow_forward
- 8. Our bodies are able to preserve a near constant pH due to the presence of hydrogen carbonate anion (HCO,). K values for the diprotic acid carbonic acid (H,CO;) are Kaj = 4.5 x 10?, K2 = 4.7 x 10-". a) Hydrogen carbonate anion is an amphoteric species. Define amphoteric. b) In the space below, write out chemical equations to describe the behavior of this species as both a Bronsted acid and a Bronsted base, and use the equilibrium constants given above to decipher whether a solution of HCO;¯ will be acidic, basic, or neutral. Support your answer with pertinent calculations.arrow_forwardThe ionization states of Phosphoric Acid are shown below: At a pH of 7.4 identify the relevant acid and conjugate base species. Using the Henderson-Hasselbach equation calculate the ratio of acid to conjugate base at this pH.arrow_forwardA chemist titrates 60.0 mL of a 0.0694M butanolc acid (HC,H.CO, solution with 0.8174M NaOH solution at 25 °C. Calculate the pH at equivalence. The pK, of butanolc acid Is 4.82. D. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of NaOH solution added. pH Ar Explanation Check 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Accessibilit hp -> esc &arrow_forward
- dont provide handwriitning solution..arrow_forwardA solution is prepared at 25 °C that is initially 0.47 Min dimethylamine ((CH,) NH), a weak base with K, =5.4 x 10 , and 0.25 M in dimethylammonium 9. chloride ((CH, NH,CI). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = |arrow_forwardA solution is prepared at 25 °C that is initially 0.051M in benzoic acid (HC,H,CO,), a weak acid with K,=6.3 × 10 °, and 0.079M in potassium benzoate (KC,H,CO,). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = [|arrow_forward
- A solution is prepared that is initially 0.41 M in trimethylamine ((CH N, a weak base, and 0.40M in trimethylammonium bromide ((CH,)¸NHB"). Complete the reaction table below, so that you could use it to calculate the pH of this solution. Use x to stand for the unknown change in [OH]. You can leave out the M symbol for molarity. [CH.),N] [CH),NH] [on] initial change final olo X O Oarrow_forwardA solution is prepared at 25 °C that is initially 0.16 M in benzoic acid (HC,H,CO,), a weak acid with K,=6.3 × 10 °, and 0.058 M in sodium benzoate -5 %3D (NaC H,CO,). Calculate the pH of the solution. Round your answer to 2 decimal places.arrow_forwardA solution is made by mixing 185 mL of 0.100 M formic acid (Ka = 1.7 x 10-4 ) with 190. mL of 0.100 M sodium formate. What is the pH of the solution? Express your answer in decimal notation rounded to three significant figures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY