
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
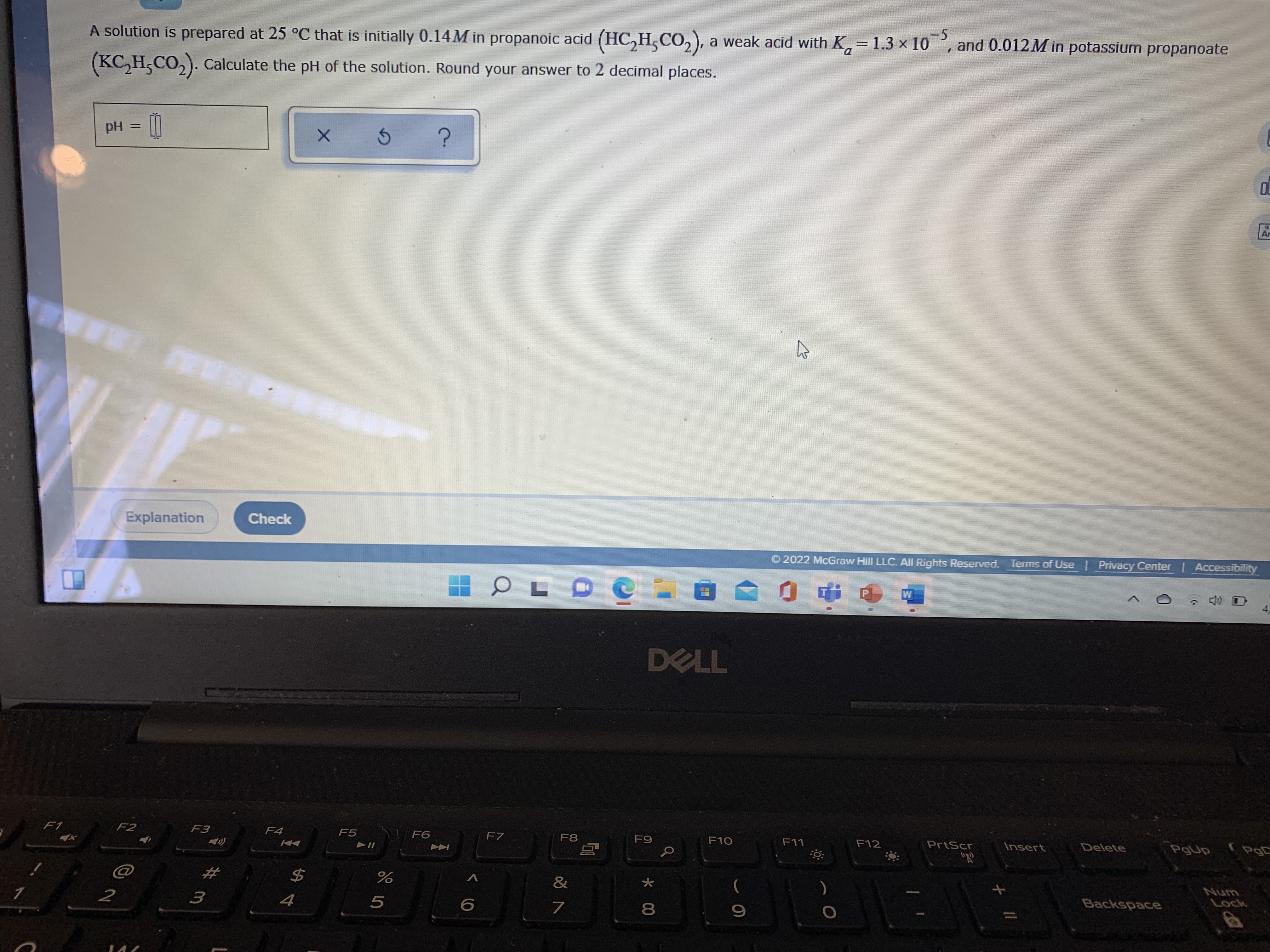
Transcribed Image Text:A solution is prepared at 25 °C that is initially 0.14M in propanoic acid (HC,H,CO,), a weak acid with K,
1.3 x 10, and 0.012M in potassium propanoate
-5
=
(KC, H,CO,). Calculate the pH of the solution. Round your answer to 2 decimal places.
pH
Explanation
Check
2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
W
DELL
F1
F2
F3
F4
F5
F6
F7
F8
F9
F10
F11
F12
PrtScr
Insert
Delete
PgUp
"R
$4
&
大
4
7
Backspace
Lock
* 00
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At 22 ˚C, an excess amount of a generic metal hydroxide, M(OH)₂, is mixed with pure water. The resulting equilibrium solution has a pH of 10.20. What is the Ksp of the salt at 22 °C? K sp= APR 9 $ 4 % mtv G Search or type URL 5 MacBook Pro < 6 ONT17 X & 7 = 11 * 00 66 8 + A ( 9 < *** m - ) O W P { + 17 [ = } 1 deletearrow_forwardA chemist titrates 230.0 mL of a 0.3332 M lidocaine (C, H,, NONH) solution with 0.1217 M HBr solution at 25 °C. Calculate the pH at equivalence. 14 21 The p K, of lidocaine is 7.94. b. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = |arrow_forwardWe want to prepare a buffered solution having a pH of 4.97 using concentrated (23.6 M) formic acid (HCOOH) and solid sodium formate (HCOONA). Ką for formic acid is 1.8 x 10-4. Incorrect. Calculate the ratio [HCOO (aq)] / [HCOOH(aq)] in the buffered solution required to achieve a pH of 4.97. [HCOO (aq)] /[HCOOH(aq)] = i 1.68E1 eTextbook and Media Hint Save for Later Attempts: 2 of 3 used Submit Answerarrow_forward
- 3b.) The Ka of acetic acid is 1.8 x 105. Calculate the pH of the following solutions: b. A solution consisting of 0.10 M acetic acid (HC2H302) and 0.50 M sodium acetate (NAC2H3O2).arrow_forwardA solution is prepared at 25 °C that is initially 0.14M in benzoic acid (HC, H, CO,), a weak acid with K=6.3 × 10 and 0.41M in potassium benzoate (KC H, CO,). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = 0arrow_forwardCalculate the pH at 25 °C of a 0.26M solution of sodium benzoate (NaC,H,Co,). Note that benzoic acid (HC,H,CO,) is a weak acid with apK, of 4.20. Round your answer to 1 decimal place. olo 18 Ar pHarrow_forward
- Calculate the pH at 25 °C of a 0.12M solution of sodium propionate (NaC,H,CO,). Note that propionic acid (HC,H,CO,) is a weak acid with a pK, of 4.89. Round your answer to 1 decimal place.arrow_forwardA chemist wishes to prepare a standard aqueous solution that has a concentration of Fe3+ equal to 1.0x10-5 M. This solution is to be prepared by allowing water at a set pH to reach equilibrium with solid Fe(OH)3. Given the fact that pH = -log [H+], what pH should be used for the aqueous solution to give this desired concentration of [Fe3+].arrow_forwardHelp neededarrow_forward
- How can we complete the data?arrow_forward4 9. 10 A solution is prepared at 25 °C that is initially 0.26M in methylamine (CH,NH,),a weak base with K, = 4.4 × 10¯*, and 0.24M in methylammonium chloride (CH,NH, CI). Calculate the pH of the solution. Round your answer to 2 decimal places. pH = 0 ? %3D Submit As Continue Terms of Use Privacy Center O 2022 McGraw Hill LLC. All Rights Reserved.arrow_forwardA solution is prepared at 25 °C that is initially 0.46M in propanoic acid (HC,H,CO,), a weak acid with K,= 1.3 x 10 - 5 and 0.15M in sodium propanoate (NaC,H,CO,). Calculate the pH of the solution. Round your answer to 2 decimal places. pHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY