
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

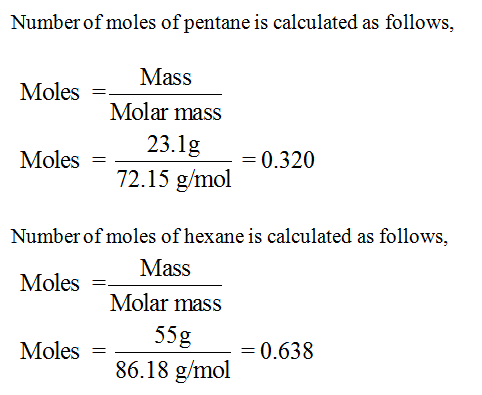
Transcribed Image Text:A solution is made by mixing 23.1g of pentane with 55.0 g of hexane at 25°C. The vapor pressure of
pure pentane and pure hexane at 25°C are 511.0 torr and 150.0 torr, respectively. What is the mole
fraction of pentane in this solution?
Expert Solution
arrow_forward
Mole fraction of pentane has to be calculated.

arrow_forward
Step 2
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Biphenyl, C12H10, is a nonvolatile, nonionizing solute that is soluble in benzene, C6H6. At 25 °C, the vapor pressure of pure benzene is 100.84 torr. What is the vapor pressure of a solution made from dissolving 19.9 g of biphenyl in 25.8 g of benzene? (anwser needs to be in torr)arrow_forwardA solution consists of 0.901 moles of toluene (C7H8) and 0.600 moles of cyclohexane (C6H12). The vapor pressures of toluene and cyclohexane at 25oC are 28.4 mmHg and 107.5 mmHg, respectively. What is the mole fraction of toluene in the vapor above this solution at 25oC?arrow_forwardA solution is made by mixing 100.0 g acetone (CH3COCH3) and 100.0 g methanol (CH3OH). a. What is the vapor pressure of this solution at 25C? b. What is the composition of the vapor expressed as a mole fraction? Assume ideal solution and gas behavior. (At 25 C the vapor pressures of pure acetone and pure methanol are 271 and 143 torr, respectively.) The actual vapor pressure of this solution is 161 torr. Explain any discrepancies.arrow_forward
- Biphenyl, C12H10, is a nonvolatile, nonionizing solute that is soluble in benzene, C6H6. At 25 ∘C, the vapor pressure of pure benzene is 100.84 Torr. What is the vapor pressure of a solution made from dissolving 11.4 g of biphenyl in 33.4 g of benzene?arrow_forwardAn electrolyte solution with a calculated vant hoff factor of 3 is prepared by dissolving 724.4 g of anunknown in enough water to make 4500 mL of solution. The osmotic pressure of this solution is113.34 atm at 30.0°C. What is the molecular weight of the unknown solute (R = 0.0821 L·atm/K·mol)The density of the solution is 0.80 g/cm³arrow_forwardA solution contains napthalene (C10H8) dissolved in hexane (C6H14) at a concentration of 10.85% napthalene by mass. Calculate the vapor pressure at 25 degrees celsius of hexane above the solution. The vapor pressure of pure hexane at 25 degrees celsius is 151 torr.arrow_forward
- A solution composed of 50.0 g strontium chloride (SrCl)) in 300 g of water at 12.0 °C. What is the mole fraction of SrCl2 in this solution? The molar mass of SrCl, is 158.5 g mol-1 and the molar mass of H2O is 18.02 g mol-1.arrow_forwardWhat is the composition of a methanol –propanol solution that has a vapor pressure of 85.7 torr at 40°C? At 40°C, the vapor pressures of pure methanol and pure propanol are 303 and 44.6 torr, respectively. Assume the solution is ideal. Mole fraction of methanol = Mole fraction of propanol =arrow_forwardA 30.0 mL sample of this aqueous HX solution freezes at – 0.186 oC. What is the molar mass of HX?arrow_forward
- 7.152g of an unknown solute was dissolved in 0.049883 kg of pure water. This solution has a boiling point of 103.4oC. The Van't Hoff factor of the unknown solute is 3. What is the Molality of the unknown? What is the Molecular weight of the unknown?arrow_forwardMenthol is a crystalline substance with a peppermint taste and odor. When 0.589 g of menthol is dissolved in 25.0 g of cyclohexane, the freezing point of the solution is lowered by 3.14 ºC. The freezing point is 6.59 and Kf constant is 20.8 for cyclohexane. Calculate the molar mass of menthol.arrow_forwardAt a certain temperature the vapor pressure of pure thiophene (C,H,S) is measured to be 0.37 atm. Suppose a solution is prepared by mixing 106. g of thiophene and 93.4 g of acetyl bromide (CH,COB1). Calculate the partial pressure of thiophene vapor above this solution. Round your answer to 2 significant digits. Note for advanced students: you may assume the solution is ideal. atmarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY