
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
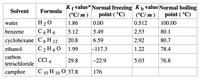
Transcribed Image Text:Kf value* Normal freezing K þ value Normal boiling
(°C/ m )
Solvent
Formula
(°C/ m )
point ( °C)
point ( °C)
H 20
С 6 Н6
water
1.86
0.00
0.512
100.00
benzene
5.12
5.49
2.53
80.1
суyclohexane C 6 Н 12
С 2 Н60
20.8
6.59
2.92
80.7
ethanol
1.99
|-117.3
1.22
78.4
carbon
CCI 4
29.8
-22.9
5.03
76.8
tetrachloride
camphor
С 10 H 160 37.8
176

Transcribed Image Text:A solution is made by dissolving 0.741 mol of nonelectrolyte solute in 857 g of benzene. Calculate the freezing point, Tf, and
boiling point, T,, of the solution. Constants can be found in the table of colligative constants.
Tf =
T, =
°C
Expert Solution
arrow_forward
Introduction
Number of moles of non - electrolyte solute = 0.741 mol
Mass of Benzene = 857 g
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A solution contains 14.6 g of the substance bolognium and 14.6 g of caterium. Assuming ideal behavior, calculate the vapor pressure of the resulting solution (in torr). (bolognium molar mass = 76.15 g mol–1 ; caterium molar mass = 58.08 g mol–1) (vapor pressures of pure substances: bolognium = 239 torr; caterium = 174 torr)arrow_forwardBiphenyl, C,,H0, is a nonvolatile, nonionizing solute that is soluble in benzene, C,H,. 10' At 25 °C, the vapor pressure of pure benzene is 100.84 Torr. What is the vapor pressure of a solution made from dissolving 13.1 g of biphenyl in 34.3 g of benzene? Psolution = Torrarrow_forwardThe freezing point of 59.49 g of a pure solvent is measured to be 44.96 ºC. When 2.18 g of an unknown solute (assume the van 't Hoff factor = 1.0000) is added to the solvent the freezing point is measured to be 42.65 ºC. If the freezing point depression constant of the pure solvent is 7.66 ºC·kg solvent/mol solute calculate the molar mass of the solute. ?=g/molarrow_forward
- Please don't provide handwritten solution ...arrow_forwardThe freezing point of a solution that contains 1.1 g of an unknown compound, (A), dissolved in 12 g of benzene is found to be 2.97 °C. The freezing point of pure benzene is 5.48 °C. The freezing point depression constant of benzene is 5.12 °C/molal. What is the molecular weight of the unknown compound?arrow_forwardHelp with the following questionarrow_forward
- A solution is made by dissolving 0.517 mol of nonelectrolyte solute in 839 g of benzene. Calculate the freezing point, T;, and boiling point, T,, of the solution. Constants can be found in the table of colligative constants. Tf = °C T, = °Carrow_forwardAt a certain temperature the vapor pressure of pure heptane C7H16 is measured to be 178. torr. Suppose a solution is prepared by mixing 110. g of heptane and 90.9g of benzene C6H6.arrow_forwardThe freezing point of pure benzene is 5.50^o C. A solution is made by dissolving 0.300 g of naphthalene, C10H8, in 15.00 g of benzene. Calculate the freezing point of this solution. Calculate the molarity of naphthalene in the resulting solution.arrow_forward
- A general chemistry student isolated ciguatera toxin (a poisonous polyether produced by the dinoflagallete Gambierdiscus toxicus) from a red tide bloom off the coast of San Francisco, California. A partial structure of ciguatera toxin is shown below. The first steps in characterizing new substances typically involve determining physical properties. In a freezing point experiment, a 174.0 mg sample of pure ciguatera toxin was dissolved in 2.075 g of diphenylether (which has a freezing point = 26.8400 °C and a freezing point depression constant = 8.000 °C/m). The freezing point of the solution was observed to be 26.3302 °C. From this experiment, calculate the molar mass of ciguatera toxin.arrow_forwardMenthol is a crystalline substance with a peppermint taste and odor. When 0.555 g of menthol is dissolved in 25.0 g of cyclohexane, the freezing point of the solution is lowered by 2.96 C. Look up the freezing point and Kf constant for cyclohexane in the Colligative Constants table. Calculate the molar mass of menthol. g/mol molar mass:arrow_forwardCalculate the boiling point of a 8.00 m aqueous solution of ethylene glycol. Boiling point constants can be found in the list of colligative constants.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY