
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![A solution has [OH]= 1x10-13.
What is the concentration of H30* ion?
[H3O*]=[
mol/L](https://content.bartleby.com/qna-images/question/2c2bb07f-2ff1-461d-826f-37518260d9fb/7f974bb2-0455-4bbc-8784-acfda0fec5dc/1lm0tvd_thumbnail.png)
Transcribed Image Text:A solution has [OH]= 1x10-13.
What is the concentration of H30* ion?
[H3O*]=[
mol/L
Expert Solution
arrow_forward
Step 1
Answer of this question:-
The multiplication of concentration of [H3O+] and [OH-] always equal to kw.
So,to find missing concentration we just have to divide kw to the known concentration.
In this case we can estimate the concentration of [H3O+] by the formula-
arrow_forward
Step 2
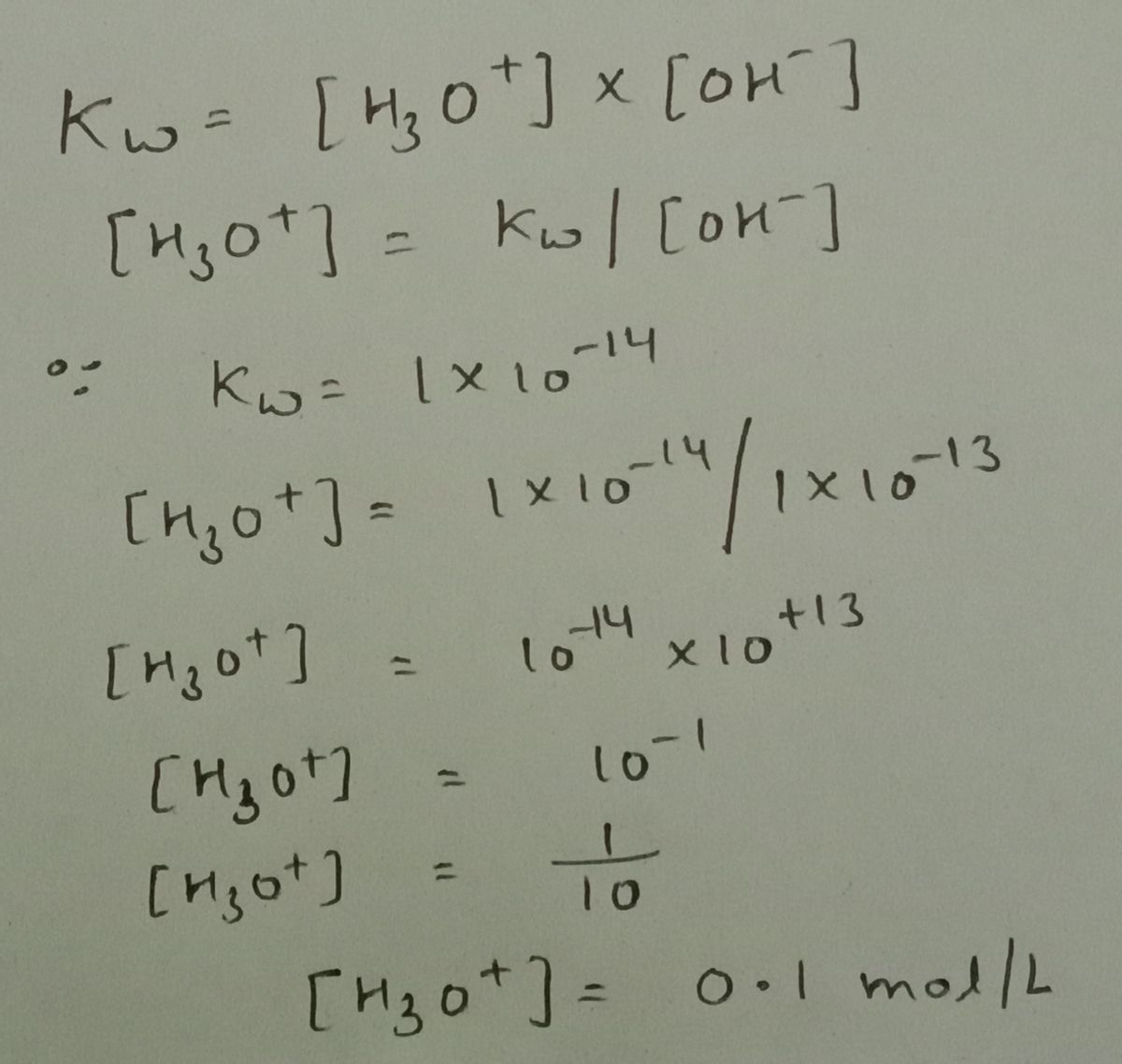
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At 25 °C, what is the hydroxide ion concentration, [OH-], in an aqueous solution with a hydrogen ion concentration of [H] 2.6 x 10-7 M? [OH-] = Marrow_forwardNonearrow_forwardWhich of the following shows the correct relationship between the ions in aqueous solutions at 25°C? ) [H30*1×[OH¯] = 1.0 x 10-7 2) [H,o"] + [OH] = 1.0 x 10-14 3 [H30"]< [OH] = 1.0 x 10-14 4 [H,0"] ÷ [OH] = 1.0 x 10-14 5 [H30"] x [OH] = 1.0 x 10-14arrow_forward
- A solution with the [OH-] = 1.00 x 10-11 has a [H+] of?arrow_forwardCalculate either H,O+ or OH| for each of the solutions at 25 °C. Solution A: [OH¯] = 2.91 x 10" M; [H,O*] = %3D Solution B: [H,0*] = 9.11 x 10-° M; [OH ] = M %3D %3D M Solution C: [H,0*] = 7.25 × 10-4 M; [OH ] = %3D %3D Which of these solutions are basic at 25 °C? Solution B: [H,O*] = 9.11 x 10-9 M %3D Solution C: [H,O*] = 7.25 x 10-4 M Solution A: [OH-] = 2.91 × 10-7 M %3D | contact us help privacy policy terms of use abeut us careersarrow_forward[H3O*) [OH] [H;O*) 1 x 10-14 2.3x10-2M %3D 4.3 x 10-13M [H3O*] Part C Calculate (H3O+] in the following aqueous solution at 25 °C: [OH-] = 6.9×10-12 M . Express your answer using two significant figures. ? Πνα ΑΣφ M H3O*] = Submit Request Answer Part D Classify the solutions as acidic or basic. Drag the appropriate items to their respective bins. P Pearson Copyright © 2022 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy Pe MacBook Air 888 DII DD F9 %23 %24 2 3 5 8 W E R Y F H J C Varrow_forward
- f the pOH of a solution is 10.59, what is the H3O+ concentration in the solution? Write the answer in scientific notation with 2 sig.dig. For example if your answer is 3.49x10^3, write down 3.49e+3. The +/- signs are important! Also, make sure there are no spaces between values, otherwise the answer will be considered incorrect!arrow_forwardCalculate the hydroxide ion concentration for a solution with [H3O+]eq = 2.8 × 10-5 M. Use "E" for scientific notation. i Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5 for 2.5 x 105)arrow_forwarda) Find the [OH–] in a solution if the [H+] is 4.0 molar. b) Find the [OH–] if the [H+] is 1.0 × 10–8 mol/L.arrow_forward
- What is the pOH of the solution with a hydroxyl concentration [OH-] 2.98 x 10-2?arrow_forwardAt 25 °C, what is the hydroxide ion concentration, [OH-], in an aqueous solution with a hydrogen ion concentration of [H+]=1.6x10-7 M?arrow_forwardAt 25 °C, what is the hydroxide ion concentration, [OH ], in an aqueous solution with a hydrogen ion concentration of [H*]=1.2 x 10-9 M? [OH"] = Xid-10 - TOOLS M X10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY