
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:earch
conds.
Question Completion Status:
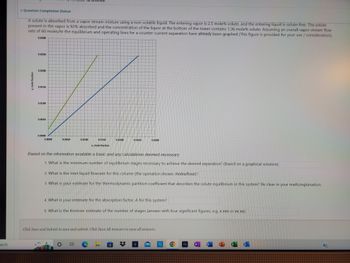
A solute is absorbed from a vapor stream mixture using a non-volatile liquid. The entering vapor is 2.5 mole% solute, and the entering liquid is solute-free. The solute
present in the vapor is 92% absorbed and the concentration of the liquor at the bottom of the tower contains 1.36 mole% solute. Assuming an overall vapor stream flow
rate of 60 moles/hr the equilibrium and operating lines for a counter-current separation have already been graphed (This figure is provided for your use / consideration).
0.0300
0.0250
0.0200
0.0150
0.0100
0.0050
0.0000
0.0000
0.0050
0.0100
0.0250
0.0300
0.0150
0.0200
x, mole fraction
Based on the information available a basic and any calculations deemed necessary:
1. What is the minimum number of equilibrium stages necessary to achieve the desired separation? (Based on a graphical solution)
2. What is the inlet liquid flowrate for this column (the operation shown; moles/hour)?
3. What is your estimate for the thermodynamic partition coefficient that describes the solute equilibrium in this system? Be clear in your math/explanation.
4. What is your estimate for the absorption factor, A for this system?
5. What is the Kremser estimate of the number of stages (answer with four significant figures, e.g. X.XXX or XX.XX)
N
W
P
X
y, mole fraction
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
E
201
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 12 images

Knowledge Booster
Similar questions
- water in a 25-mm-diameter test tube evaporating into dry air at 1 atm. The distancefrom the water-air interface to the top of the tube is L=15 cm. The mass fraction of the watervapor at the water-air interface is 0.0235, and the binary diffusivity of water vapor in air is2.6x10-5 m2/s.a) the mass evaporation rate of the waterb) the water vapor mass fraction at x=L/2.c) Plot the water vapor mass fraction from x = 0 to x=Ld) the fraction of the water mass flow that is contributed by bulk flow and thefraction contributed by diffusion at x=L/2.arrow_forwardExample 5.1. an engineer in the chemical plant, you are asked to design an acetonerecovery system having the flow sheet illustrated on the next page. In this Example, to make the calculation simple, all concentrations of both gases and liquids are in presented in weight (mass) percent (please note, Acetone vapour is considered toxic to the environment (as well as human health). As however, that, normally, for gas phase, the concentration is presented in “volume“ and “mole“ basis) Calculate the values of A, F, W, B, and D in kg per hour, if given G = 1,400 kg/h water (100%) w Ka/h Air tA ka/h Air 99.5 water O.5% Condenser Distillattet D kO/ Aceton e 99.0% water 1.0% Absorber Distillation column 2) column -Bottom ka/h Acetone 19.09% water 81.0% B kg/h Acetone 4.0% water 96.0% Feed G kg/h Acetone 3.0% Air 95.0% Water 2.0%arrow_forwardAn input stream contains oil sands, comprising 14.0% oil, the remainder being solid sand particles. In the initial stages of steady-state processing, the oil sands are mixed with warm water and pumped to a settling tank. In this tank three layers are formed. The three layers are: 1. Bottom layer containing 95.0% sand and 5.00% water 2. Middle layer containing 5.00% oil and 95.0% water 3. Top layer containing 70.0% oil and 30.0% water The top layer is skimmed off and sent for further processing, while the bottom two layers are disposed of. How much warm water is added to 200 tonnes of oil sands in this process if 85.0% of the total oil is recovered in the top layer (3 sig. figs)? All percentages given are weight percentages.arrow_forward
- Benzene is to be absorbed from coal gas by means of a wash-oil. The inlet gas contains 3 per cent by volume of benzene, and the exit gas should not contain more than 0.02 per cent benzene by volume. The suggested oil circulation rate is 480 kg oil/100 m³ of inlet gas measured at 273 K and 101.3 kN/m². The wash oil enters the tower solute-free. If the overall height of a transfer unit based on the gas phase is 1.4 m, determine the minimum height of the tower which is required to carry out the absorption. The equilibrium data are: Benzene in oil (per cent by mass) Equilibrium partial pressure of benzene in gas (kN/m²) 0.05 0.013 0.01 0.033 0.50 0.20 1.0 0.53 2.0 1.33 3.0 3.33arrow_forwardIn a counter-current absorption tower, a process gas containing 0.04 (mole fraction) ammonia is scrubbed with pure water. The exit gas contains 0.002 (mole fraction) ammonia.The diameter of the tower is 1m, and the packing has a large area per volume of 200 m2m–3. The overall mass transfer coefficient based on the liquid side driving force, KL, is 1.5×10−5ms–1. The total gas and liquid concentrations are 0.6kmolm–3and 20 kmolm–3respectively and the gas flowrateis 0.03kmols–1. The actual liquid to gas ratio is 1.5 times that of the minimum liquid to gas ratio. The equilibrium for ammonia between the air and water is given by y* = 1.04x. What is the mol fraction of ammonia in the liquid?arrow_forward1) An evaporator is concentrating solutions coming from three different sources.The first source contains 20% NaCl by weight, the rest is water. The secondsource contains 10% by weight NaCl, 30% NaOH by weight, the rest water. Thethird source contains 25% NaCl by mole, 25 % NaOH by mole, the rest water.The feed streams are fed directly to the evaporator at the following flow rates;100 kg/min for the first source, 7800 kg/min for the second source and 120kg/min for the third source. If 70% of the original water is evaporated, calculate(a) composition in % by weight of the product (b) the flow rate in kg/min of theproduct.2) Two process streams are mixed to form a single stream. Only the flow in themixed stream is known. A soluble salt is added to one of the original streams ata steady rate. Samples taken of this stream show it to be 4.76 % w salt. Samplesfrom the combined stream show 0.62% w salt. What is the ratio of the flows inthe two original streams?3) A chemist attempts to prepare…arrow_forward
- A cooling tower works with the amount of cooling water 1 ton/hour. Conditions of cooling water in the cooling tower has a solid content of 1350 ppm. The cooling water entering the cooling tower is processed in such a way that the solids content is a maximum of 225 ppm. If the cooling water evaporated rate is assumed to be 2.5% the cooling water inlet rate, calculate the concentration cycle, the added water rate, and the blowdown water rate!arrow_forwardA reverse-osmosis unit is used to obtain pure water from saline water. The coefficient of water permeation is 0.1 mol/cm2 s atm. The pressure differential across the membrane is 0.2 atm, and the osmotic pressure differential is 0.05 atm. The water flux across the membrane is most nearly (A) 0.0050 mol/cm2·s (B) 0.015 mol/cm2·s (C) 0.050 mol/cm2·s (D) 0.15 mol/cm² ·sarrow_forwardA stream containing propionic acid and water at 60:40 mass ratio is fed to a mixer at 3 kg/s with pure cyclohexane as solvent. What should be the flow rate of the solvent in order to attain 40:60 mass ratio of solute and solvent in the extract (solvent-rich) stream? Prepare graphical and computational solution.arrow_forward
- You are working on determining the Oxygen Transfer Rate (OTR) and Oxygen Uptake Rate (OUR) in a bioreactor. You perform two set of experiments in a 3L bioreactor. During the first set of experiments, you fill the bioreactor with water only, you remove all the dissolved oxygen and start sparging it with air at the specified agitation speeds and air flowrate. Which of the following methods could be used to estimate the volumetric oxygen transfer coefficient (kLa)? C*: saturation concentration of dissolved Oxygen CL: concentration of dissolved Oxygen at any given time Question options: kLa is the slope of a ln(C* ) versus time plot kLa is the slope of a CL versus time plot -kLa is the slope of a ln(C* - CL) versus time plot kLa is the slope of a ln(C* - CL) versus time plotarrow_forwardA water cleanup operation involves stripping vinyl chloride from contaminated ground water at 25 °C and 850 mm. Hg using a countercurrent, staged stripper. The feed has 5.0 ppm(molar) vinyl chloride, and the outlet water contains 0.1 ppm (molar) vinyl chloride. Inlet air used for stripping is pure. For a liquid flow rate of L = 1 kmol/h , determine the following : Minimum gas flow rate in kmol/h. If the actual gas flow rate = 2.0 × (minimum gas flow rate) calculate the number of stages Henry’s constant (HB) for vinyl chloride = 1243. 84 atm.arrow_forward1. [25] The diffusion of the gas phase A through a layer follows the equation at dz² Solve the differential equation either by the variable combination method variable separation or Laplace transform, Choose a method that you understand, if you know the initial conditions and boundary conditions are as follows: C (z, 0) = Co C (0, t) = Cs C (0, t) = 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The