
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Classify the molecules in Problems 26.43 and 26.48 as either carbon-chain or heterochain

Transcribed Image Text:(a) Show how poly(propylene oxide) can be synthesized via anionic ring-opening polymerization. (b) Draw the mechanism
for the initiation and first two propagation steps.
Poly(propylene oxide)
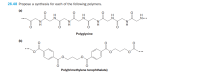
Transcribed Image Text:26.48 Propose a synthesis for each of the following polymers.
(a)
H
N...
Polyglycine
(b)
Poly(trimethylene terephthalate)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a) Write the structural formula of aspirin (acetylsalicylic acid), and indicate the carboxyl groups and ester in the formula Iarrow_forwardthe monomer units of amylose, the main component of starch, andglycogen, the animal stored carbohdrate, are both composed of a a-D-glucose monomers. however, the structural differences between these two polymers are remarkable: amylose is a coiled single stranded polysaccharaide while glycogen forms long branched networks. Explain the difference.arrow_forwardHow many carbon atoms are in the longest chain? number of carbon atoms:arrow_forward
- Which polymer has an alkene functional group? A) Nomex B) Polyvinyl acetate C) Rubber D) Polyacrylate E) Polyethylene glycolarrow_forwardPlease,Don't provied handwriting solution..arrow_forwardIf it takes 67 mL of 3 M KOH to neutralize 480 mL of sulfuric acid (H2SO4) solution, what is the concentration of the H2SO4 solution?arrow_forward
- Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true - false status of the statements using the choices. (1) Alcohols have higher boiling points than alkanes of similar molecular mass because of hydrogen bonding. (2) Polyols have similar boiling points to tertiary alcohols. (3) Primary and secondary alcohols give the same type of product when subjected to mild oxidizing agents. Group of answer choices All three statements are true. Two of the three statements are true. Only one of the statements is true. None of the statements are true.arrow_forwardCharles Goodyear discovered that if sulfur is added to rubber and the mixture is heated, the rubber hardens, becomes more resilient, and does not melt. This process is referred to as and involves the formation of sulfur bridges between the methyl side groups on different chains. A polymerization sintering c hybridization D linking E) vulcanizationarrow_forwardHow do you assign priority in a ring structure when determining if a carbon within the ring is (R) or (S)?arrow_forward
- explain the structural isomerism and stereoisomerismarrow_forwardRecognize fused aromatic systems such as polynuclear aromatic hydrocarbons andfused heterocyclic compounds, and use the theory of aromatic compounds to explaintheir properties.arrow_forwardWhat are the repeating units of the polymer that would form if themolecule was polymerized?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY