
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
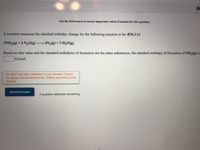
Transcribed Image Text:Use the References to access important values if needed for this question.
A scientist measures the standard enthalpy change for the following reaction to be -876.3 kJ:
2NH3(g) +3 N20(g)·
4N2(g) + 3 H2O(g)
Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of NH3(g) is
kJ/mol.
An error has been detected in your answer. Check
for typos, miscalculations etc. before submitting your
answer.
Submit Answer
5 question attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described by the following equation: Fe2O3(s)+3CO(g)2Fe(s)+3CO2(g)H=24.8kJ The enthalpy change for the combustion of carbon monoxide is 2CO(g)+O2(g)2CO2(g)H=566kJ Use this information to calculate the enthalpy change for the equation 4Fe(s)+3O2(g)2Fe2O3(s)H=?arrow_forwardWhat mass of acetylene, C2H2(g), must be burned to produce 3420 kJ of heat, given that its enthalpy of combustion is 1301 kJ/mol? Compare this with the answer to Exercise 5.91 and determine which substance produces more heat per gram.arrow_forward9.73 Without looking up any numerical data or doing calculations, predict whether the enthalpy change for each of the following reactions should he positive, negative, or zero. (a) H2O(l)H2O(s) (b) N2(g)2N(g) (c) CH4(g)+2O2(g)CO2(g)+2H2O(l) (d) CO2(s)CO2(g)arrow_forward
- Compounds with carboncarbon double bonds, such as ethylene, C2H4, add hydrogen in a reaction called hydrogenation. C2H4(g)+H2(g)C2H6(g) Calculate the enthalpy change for this reaction, using the following combustion data: C2H4(g)+3O2(g)2CO2(g)+2H2O(l);H=1411kJC2H6(g)+72O2(g)2CO2(g)+3H2O(l);H=1560kJH2(g)+12O2(g)H2O(l);H=286kJarrow_forwardWhen 2.50 g of methane burns in oxygen, 125 kJ of heat is produced. What is the enthalpy of combustion per mole of methane under these conditions?arrow_forwardUsing the data in Appendix G, calculate the standard enthalpy change for each of the following reactions: (a) Si(s)+2F2(g)SiF4(g) (b) 2C(s)+2H2(g)+O2(g)CH3CO2H(l) (c) CH4(g)+N2(g)HCN(g)+NH3(g) ; (d) CS2(g)+3Cl2(g)CCl4(g)+S2Cl2(g)arrow_forward
- Given the following (hypothetical) thermochemical equations: A+B2C;H=447kJA+3D2E;H=484kJ2D+B2F;H=429kJ Calculate H, in kJ, for the equation 4E+5B4C+6Farrow_forwardWhich of the enthalpies of combustion in Table 5.2 the table are also standard enthalpies of formation?arrow_forwardIn a calorimetric experiment, 6.48 g of lithium hydroxide, LiOH, was dissolved in water. The temperature of the calorimeter rose from 25.00C to 36.66C. What is H for the solution process? LiOH(s)Li(aq)+OH(aq) The heat capacity of the calorimeter and its contents is 547 J/C.arrow_forward
- In a bomb calorimeter, the reaction vessel is surrounded by water that must be added for each experiment. Since the amount of water is not constant from experiment to experiment, the mass of water must be measured in each case. The heat capacity of the calorimeter is broken down into two parts: the water and the calorimeter components. If a calorimeter contains 1.00 kg water and has a total heat capacity of 10.84 kJ/C, what is the heat capacity of the calorimeter components?arrow_forwardHow much heat is absorbed by a 44.7-g piece of leadwhen its temperature increases by 65.4°C?arrow_forward9.41 Under what conditions does the enthalpy change equal the heat of a process?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning