
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:$
.
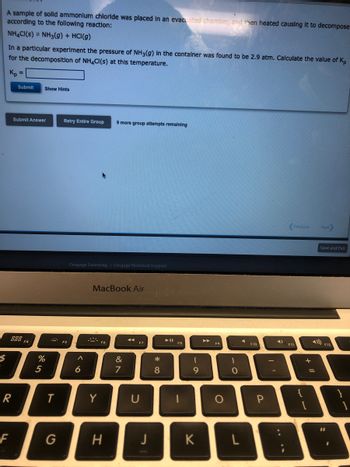
A sample of solld ammonium chloride was placed in an evacuated chamber, and then heated causing it to decompose
according to the following reaction:
NH4CI(S) NH3(g) + HCl(g)
In a particular experiment the pressure of NH3(g) in the container was found to be 2.9 atm. Calculate the value of K₂
for the decomposition of NH4CI(s) at this temperature.
Kp =
R
F
Submit Show Hints
Submit Answer
888 F4
%
5
T
G
Retry Entire Group 9 more group attempts remaining
F5
Cengage Learning Cengage Technical Support
A
6
MacBook Air
Y
F6
H
&
7
◄◄
F7
U
J
* 00
8
► 11
F8
1
6a
K
►►
F9
O
)
O
F10
P
4)
Previous Next
F11
+ "
Save and Exit
Expert Solution
arrow_forward
Step 1
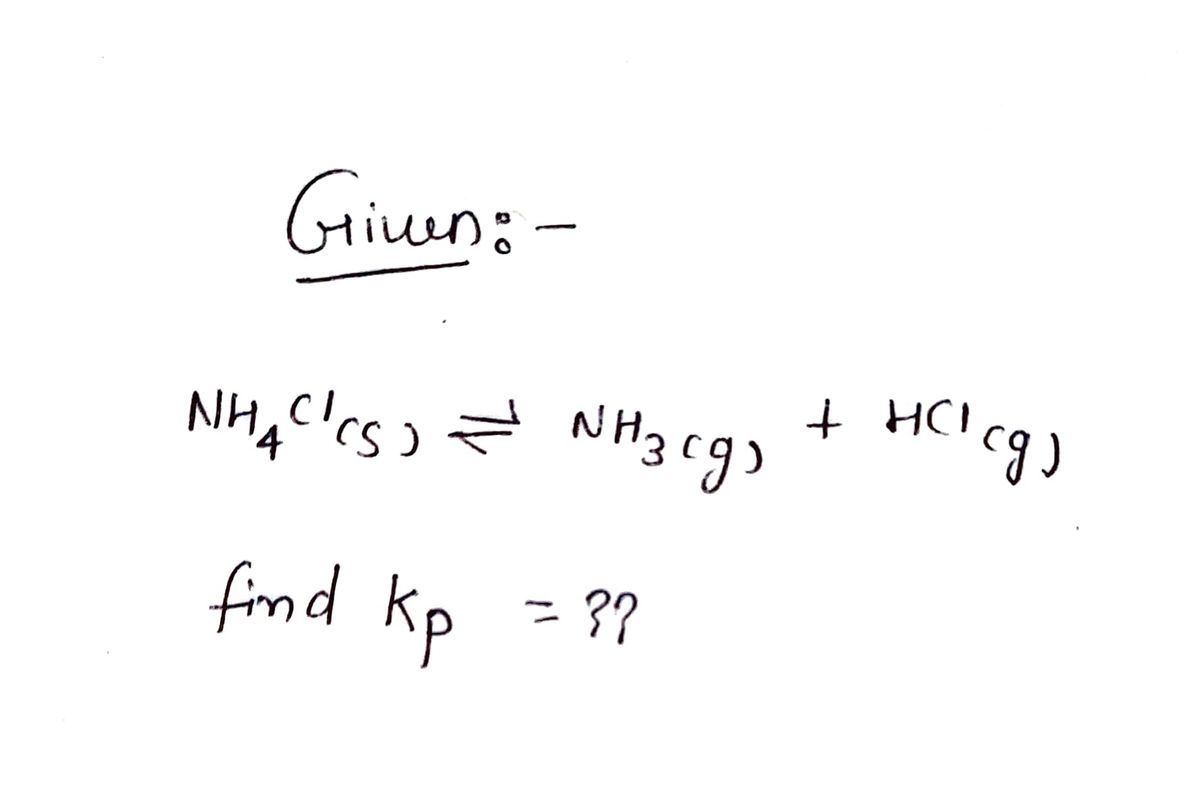
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The equilibrium constant Kp for the reaction CCl4(g) —> C(s) + 2Cl2(g) at 700˚C is 0.76. Determine the initial pressure of carbon tetrachloride that will produce an equilibrium pressure of 1.5 atm for Cl2 at 700˚C. (Since C is a solid it can be left out of the ICE chart.)arrow_forwardConsider the following reaction:2HI(g) ⇄ H2(g) + I2(g)At 500 K, the equilibrium constant, Kp is 0.049. A 1.50 L container at 500 K is initially filled with 0.809 mol of HI and allowed to equilibrate. What is the equilibrium concentration of H2(g)?arrow_forwardKp for the following reaction at a give temperature is 0.263 at 1000. K: C(s) + 2 H2(g) = CH4(g) What is the total pressure (in atm) in a 10.0 L flask when 25.0 g of C(s) and 4.50 g of H2 are introduced to the flask and allowed to equilibrate at 1000. K? Enter your answer without units.arrow_forward
- The value of Kp for the reaction 2 A(g) + B(g) + 3 C(g) → 2 D(g) + E(g) is 12770 at a particular temperature. What does the magnitude of Kp tell us about the equilibrium position of the reaction? A) There are many more products than reactants. B) There are only a few more products than reactants. C) There are many more reactants than products. D) There are only a few more reactants than products.arrow_forwardAt 1560 oC the equilibrium constant for the reaction: 2 IBr(g) I2(g) + Br2(g) is KP = 0.846. If the initial pressure of IBr is 0.00674 atm, what are the equilibrium partial pressures of IBr, I2, and Br2?p(IBr) = p(I2) = p(Br2) =arrow_forwardConsider the equilibrium system described by the chemical reaction below. If the partial pressures at equilibrium of NO, Cl2, and NOCI are 0.095 atm, 0.171 atm, and 0.28 atm, respectively, in a reaction vessel of 7.00 L at 500 K, what is the value of Kp for this reaction? 2 NO(g) + Cl2(g) = 2 NOCI(g)arrow_forward
- Some quantity of NOBr is added to an otherwise empty flask. The reaction: 2 NOBr (g) ⇄ 2 NO (g) + Br2 (g) takes place at a particular temperature for which KP is 3.5 x 10-5. Assuming an equilibrium pressure of NOBr = 2.46atm, what is the equilibrium pressure of NO?arrow_forwardAt 500K, the methanol decomposes according to the following reaction, CH3OH(g) = CO(g) + 2 H2(g) K= 9.60×10-2 Some methanol, CH3OH (molar mass: 32 g/mol), is placed into a rigid 2.00-L vessel, and the temperature is raised to 500K. If the concentration of H2 in the equilibrium mixture is 0.246M, what is the initial concentration of methanol? O A, 0.231 M Ов. 0.866 М OC 0.200 M OD. 1.109 M O E. 0.012 Marrow_forwardThe value of K, for the reaction 2 NO, (g) N204 (g) is 1.52 at 319 K. What is the value of the Kp of the reaction NO2 (g) 1/2 N204 (g) at this temperature: 0.761 O2.31 1.23 1.52 0.811 A Moving to another question will save this response. Esc AI C 中 F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 23 %24arrow_forward
- The equilibrium constant Kp for the reaction PC15(g) = PC13(g) + Cl₂(g) is 1.05 at 250°C. The reaction starts with a mixture of PC15, PC13, and Cl₂ at pressures 0.177 atm, 0.233 atm, and 0.161 atm, respectively, at 250°C. When the mixture comes to equilibrium at that temperature, which pressure(s) will have decreased? Cl₂ PC13 PC15arrow_forwardAt 25 °C, the following concentrations were found for the gases in an equilibrium mixture for the reaction N₂O(g) + NO₂(g) = 3NO(g) [NO₂] = 0.75 M, [NO] = 9.0 × 10-8 M, and [N₂O] = 0.033 M. What is the value of Kp for this reaction? Enter your answer in scientific notation. Kp= i x 10 iarrow_forwardKp is equal to 25.00 at some temperature for the reaction: H2(g) + I2(g) ⇌ 2 HI(g). Clair and Pat put the gases in a sealed container with 0.1000 atm each of H2 and I2; and 1.0000 atm of HI, then waited for the system to reach equilibrium several hours later. Calculate the Reaction Quotient giving the starting concentrations.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY