
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
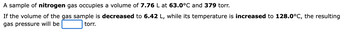
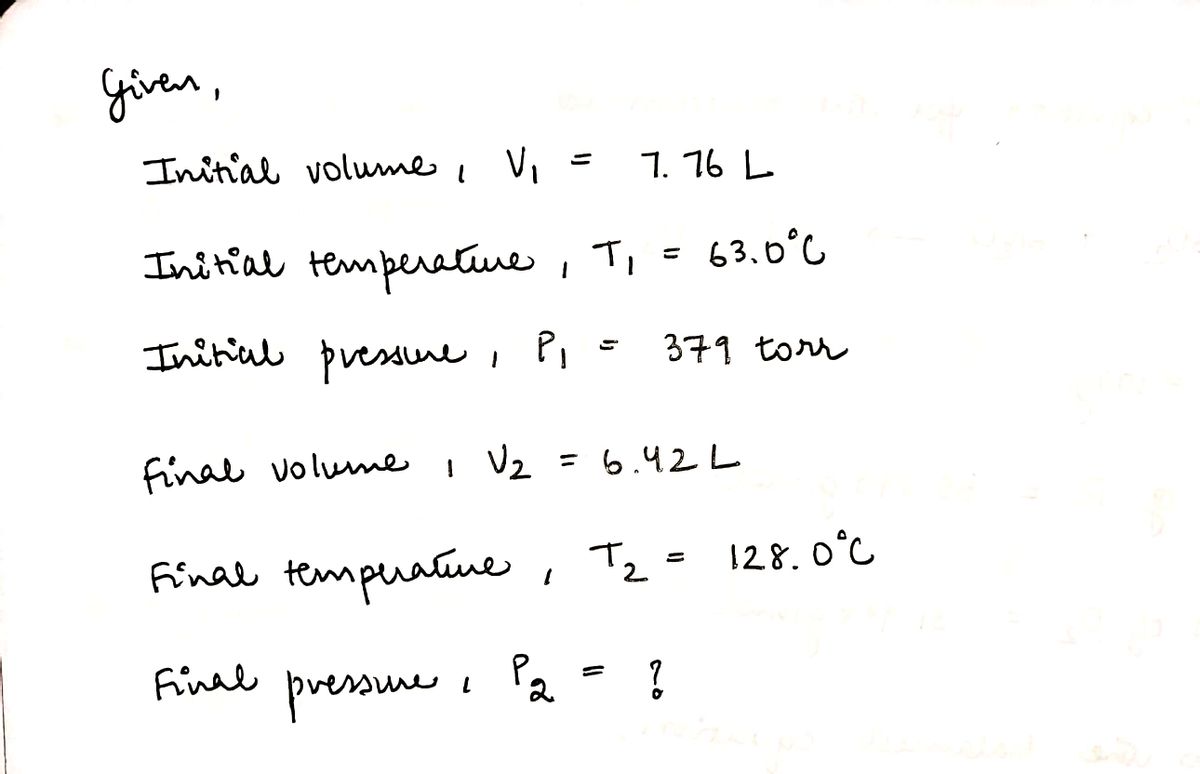
Transcribed Image Text:A sample of nitrogen gas occupies a volume of 7.76 L at 63.0°C and 379 torr.
If the volume of the gas sample is decreased to 6.42 L, while its temperature is increased to 128.0°C, the resulting
gas pressure will be
torr.
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of carbon dioxide gas occupies a volume of 3.40 L at STP (0oC and 1.0 atm). What volume will the same sample occupy if the temperature is increased to 40.0oC and the pressure is changed to 460 mmHg?arrow_forwardA mixture of oxygen and krypton gases, in a 7.91 L flask at 41 °C, contains 8.74 grams of oxygen and 7.22 grams of krypton. The partial pressure of krypton in the flask is atm and the total pressure in the flask is atm.arrow_forwardThe density of a gas is 1.74 g/L at 31oC and 592 torr. Calculate the molar mass of this gas. R = 0.08206 L atm/mol Karrow_forward
- If 1.52 g Ar are added to 3.41 atm He in a 2.00 L cylinder at 27.0 °C, what is the total pressure of the resulting gaseous mixture? Ptotal = atmarrow_forwardA sample of neon gas occupies a volume of 7.00L at 68.0 degree celcius and 393 torr. If the volume of the gas sample is increased to 9.76 L,while its temperature is increased to 123.0 degree celcius,the resulting gas pressure will be ____torr.arrow_forward1. A 0.678 gram sample of gas occupies 0.214 L at standard conditions; what is the molar mass of the gas? 2. A sample of gas occupies 250. mL at 37°C and 730, torr; what volume would the gas occupy at standard conditions? 3. A 5.00 mL sample of an unknown liquid is vaporized in a flask having a volume of 285 mL. At 100°C, 0.4168 g of the vapor exerts a pressure of 740 torr. Calculate the gram molar mass of the unknown liquid. 4. Calculate the density of oxygen gas at 50°C and 750. torr. 5. Calculate the density of H2S at STP. 86arrow_forward
- A mixture of argon and krypton gases, in a 9.30 L flask at 46 °C, contains 6.46 grams of argon and 45.4 grams of krypton. The partial pressure of krypton in the flask is atm and the total pressure in the flask is atm.arrow_forwardA 3.50 L flask was used to collect at 7.85 g sample of propane gas, C3H8. After the sample was collected the gas pressure was found to be 752 mmHg. What was the temperature of the propane in the flask?arrow_forwardIn a gas mixture, the partial pressures are nitrogen 0.559 atm, oxygen 115 torr, and helium 0.296 mm Hg. What is the total pressure (in torr) exerted by the gas mixture?arrow_forward
- A mixture contains O2 at 700. torr pressure, F2 at 600. torr pressure, and Cl2 at 400. torr pressure. What is the total pressure of the gases in the system?arrow_forwardA gas occupies 547 mL at 596.0 Torr and 26.9 °C. When the pressure is changed to 326.5 Torr, what temperature is needed to maintain the same volume?arrow_forwardA sample of helium gas occupies a volume of 6.79 L at 60.0°C and 391 torr. If the volume of the gas sample is decreased to 4.61 L, while its temperature is increased to 131.0°C, the resulting gas pressure will be torr.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY