
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
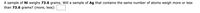
Transcribed Image Text:A sample of Ni weighs 73.6 grams. Will a sample of Ag that contains the same number of atoms weigh more or less
than 73.6 grams? (more, less):
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sample of MGSO4 x NH2O contains 7.81 grams of MGSO4 and 8.91 grams of H2O, What is the value of N?arrow_forward9. How many hydrogen atoms are there in 500.0 L of water? (d1.0 = 1.00 g mL-) %3D A: 3.34 x 1028arrow_forwardWhat is the mass, in grams, of 1.51 x 1018 atoms of gold present in one nanoparticle? i eTextbook and Media Hint Save for Later Attempts: 3 of 1 ост 4 MacBook Airarrow_forward
- In the Star Wars series, the death star is a spherical space station and weapon with a diameter of 99 miles. If we had a solid iron sphere with a diameter 5.81 times as large as the death star's diameter, how many iron atoms would be needed to construct such a sphere? (diron = 7.874 g/cm3)arrow_forwardOn earth, tin has 10 different stable isotopes. The heaviest, 124 Sn makes up 5.80% of naturally occurring tin atoms. a) How many atoms of 124 Sn are present in 42.7 g of a natural sample of tin? 124 Sn: 1.491e24 atoms Scientific Notation: 1.491 x 1024 Helpful tip: To enter a number in scientific notation, enter 0E5 or 1.0e5, for 1.0 x 105 where the E# (or e# ) denotes 10# b) Considering 124 Sn has an isotopic mass of 123.905 amu, calculate the mass of this isotope in the sample. Mass: 8.0 garrow_forwardA substance contains 35.0g nitrogen, 5.05g hydrogen and 60.0g of oxygen. How many grams of hydrogen are there in a 185g sample of the substance?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY