
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
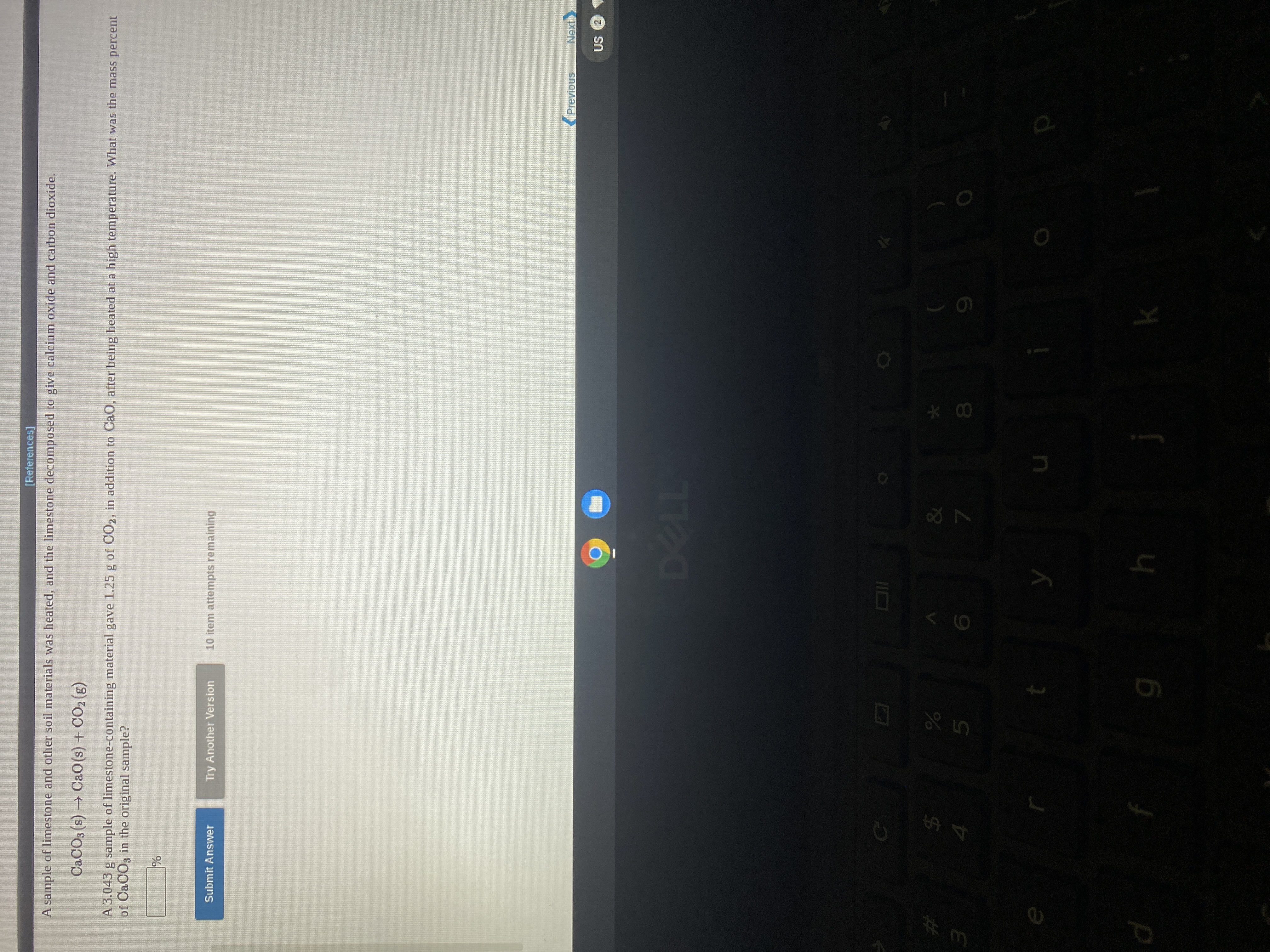
Transcribed Image Text:A sample of limestone and other soil materials was heated, and the limestone decomposed to give calcium oxide and carbon dioxide.
CACO3 (s) → Ca0(s) + CO2 (g)
A 3.043 g sample of limestone-containing material gave 1.25 g of CO2, in addition to CaO, after being heated at a high temperature. What was the mass percent
of CaCO3 in the original sample?
Expert Solution
arrow_forward
Step 1
CaCO3(s) --> CaO(s)+ CO2(g)
Mass of lime stone containing sample = 3.043g
CO2 produced = 1.25g
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Morphine (C₁₇H₁₉NO₃) is a painkiller in the opiate family. A sample of morphine was discovered that had been diluted by mixing with table salt (sodium chloride). When 2.00 g of the mixture undergoes combustion, 2.49 g of CO₂ is produced. What is the mass percent of morphine in the mixture?arrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [] → NaNO₂(aq) + H₂O(1) X Śarrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. →KClO2(aq)+H2O(l)arrow_forward
- Consider the chemical reaction that takes place between solid copper and oxygen gas. Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Cu(s) + O2(g) → 3C2 2- Reset 1 3 4 6 7 8. 9. -> (s) (1) (g) (aq) H Cu NR • x H20 LOarrow_forward4. When a 19.34 gram piece of lithium is placed in a sample of water, how many grams of hydrogen gas are? 2 Li ( + 2 H,O → 2 LİOH(aq) + H«e) (s) 2(g)arrow_forwardDetermine the mass in grams of 3.00 × 10²¹ atoms of arsenic. (The mass of one mole of arsenic is 74.92 g.) Determine the formula weight of Al₂(SO₄)₃. Provide an answer to two decimal places. How many moles of Ag are in 43.6 grams of Ag₂O? 100.0 mL of a 0.500 M solution of KBr is diluted to 500.0 mL. What is the new concentration of the solution?arrow_forward
- Predict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [] → Ba(C1O₂)₂(aq) + H₂O(1) 2 X Garrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [[] → BaCl₂(aq) + H₂O(1) X Sarrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. → CaC1,₂(aq) + H₂O(1) X Śarrow_forward
- Two solutions were made, the first one was made by dissolving 2.27 g of Co(NO3)2 in 100.0 mL of water. Then, to make the second solution, 5.00 mL of this solution was added to the 250 mL volumetric flask, which was filled to the mark with water. What was the molar concentration of the Co(NO3)2 in the two solutions?arrow_forwardA sample of limestone and other soil materials was heated, and the limestone decomposed to give calcium oxide and carbon dioxide. CACO3 (s) → Ca0(s) + CO2 (g) A 5.483 g sample of limestone-containing material gave 2.14 g of CO2, in addition to CaO, after being heated at a high temperature. What was the mass percent of CACO3 in the original sample? %arrow_forwardWrite the balanced molecular chemical equation for the reaction in aqueous solution for sodium hydroxide and tin(IV) acetate. If no reaction occurs, simply write only NR. 1 2 3 4 O 口。 ロ Dロロ:ロ|0口。 (s) (g) (aq) H. NR Na Ti Ac Snarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY