
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
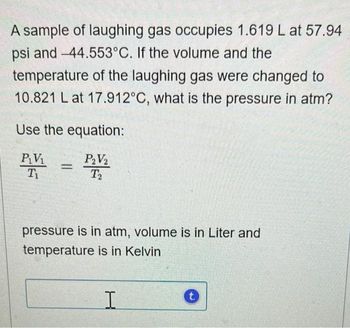
Transcribed Image Text:A sample of laughing gas occupies 1.619 L at 57.94
psi and -44.553°C. If the volume and the
temperature of the laughing gas were changed to
10.821 L at 17.912°C, what is the pressure in atm?
Use the equation:
P₂V₂
P₁V₁
Ti
T₂
=
pressure is in atm, volume is in Liter and
temperature is in Kelvin
I
t
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of carbon dioxide occupies 1.65 L at -20.0°C and 20.0 psi. If the volume of the gas is 2.65 L at 35.0 psi, what is the Celsius temperature? 438°C 711°C 551°C 824°C 984°Carrow_forwardA gas sample has a volume of 169 mL at 0.00 ∘C. The temperature is raised (at constant pressure) until the volume is 222 mL. What is the temperature of the gas sample in ∘C at this volume?arrow_forwardA sample of a gas at 13.0°C has a volume of 0.45 L at 1.1 atm. What is the volume of the gas at 25.0°C and 2.6 atm?arrow_forward
- A gas is stored in a 25.0 liter tank, at temperature of 45 degrees Celsius, and an unknown pressure. The gas is then pumped into a new tank which has a volume of 65.0 L, a temperature of 75 degrees Celsius, and a pressure of 2.09 atm. What was the original pressure of the gas (in atm)? Use 273 when converting temperature to Kelvin Do not report units in your answer. Your Answer: Answerarrow_forwardA gas mixture has a total pressure of 0.51 atm and consists of He and Ne. If the partial pressure of He in the mixture is 0.23 atm, what is the partial pressure of the Ne in the mixture? Enter your answer in the provided box. atmarrow_forwardA gas that has a volume of 8.5 liters, a temperature of 35 0C, and an unknown pressure has its volume increased to 17 liters and its temperature decreased to 130C. If I measure the pressure after the change to be 1.6 atm, what was the original pressure of the gas? If I have 3.6 liters of gas at a temperature of 36 0C and a pressure of 4.6 atm, what will be the pressure of the gas if I raise the temperature to 84 0C and decrease the volume to 2.6 liters? A tire has a pressure of 1.4 atm and 21 0C. At the end of a long trip, the tire pressure increased to 1.6 atm. What is the new temperature inside of the tire in degrees Celsius? A sample of chlorine gas is under a 1.24 atm pressure, in a 2.84 L container, at 110 0C . How many grams of chlorine are present in this sample?arrow_forward
- A sample of an ideal gas has a volume of 2.26 L at 284 K and 1.12 atm. Calculate the pressure when the volume is 1.78 L and the temperature is 301 K. ?= atmarrow_forwardA sample of an ideal gas has a volume of 3.40 L at 10.80 °C and 1.80 atm. What is the volume of the gas at 19.20 °C and 0.986 atm? V = Larrow_forwardPart B A sample of gas in a cylinder as in the example in Part A has an initial volume of 64.0 L, and you have determined that it contains 1.10 moles of gas. The next day you notice that some of the gas has leaked out. The pressure and temperature remain the same, but the volume has changed to 16.0 L. How many moles of gas (m₂) remain in the cylinder? Express your answer with the appropriate units. View Available Hint(s) Value Units 71₂=arrow_forward
- A sample of an ideal gas has a volume of 3.40 L at 14.80 °C and 1.90 atm. What is the volume of the gas at 21.40 °C and 0.993 atm? V = Larrow_forwardA gas has a temperature of 14.0 degrees C, and a volume of 4.50 liters at 1.00 atm. If the temperature is raised to 29.0 degrees C and the pressure is doubled, what would be the new volume of the gas?arrow_forwardA sample of an ideal gas has a volume of 3.40 L at 14.60 °C and 1.90 atm. What is the volume of the gas at 23.80 °C and 0.990 atm? V = Larrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY