
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
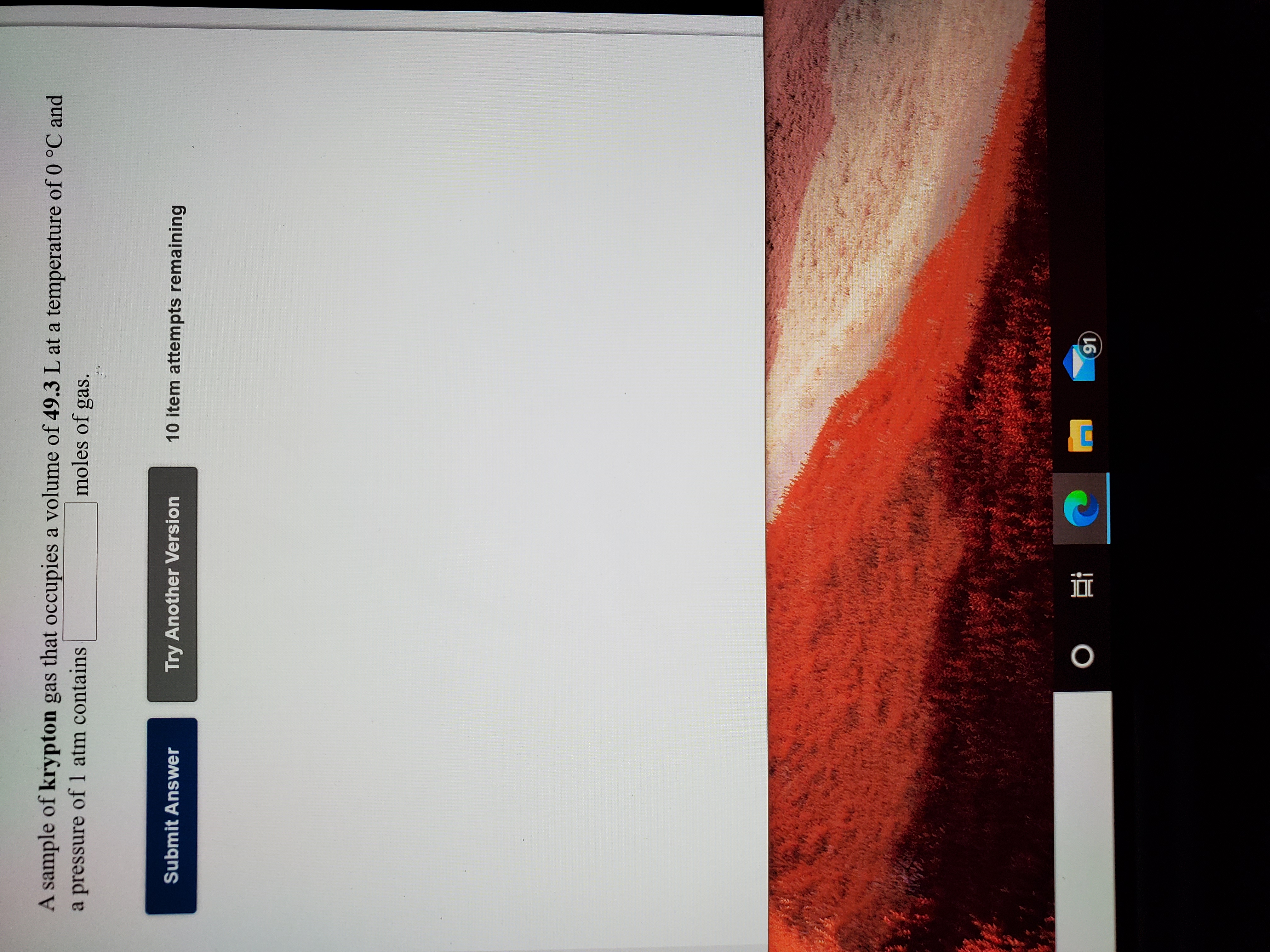
Transcribed Image Text:A sample of krypton gas that occupies a volume of 49.3 L at a temperature of 0 °C and
a pressure of 1 atm contains
moles of gas.
Submit Answer
Try Another Version
10 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- TUTOR Determining the Density of a Gas Calculate the density of nitrogen gas (in g/L) at 518 mmHg and 51.1 °C. g/L Submit Show Approach Show Tutor Steps Submit Answer Try Another ersion FLV Eo search 8.arrow_forward1 pt 1 pt L A sealed balloon is filled with 1.70 L helium at 20.°C and 1.40 atm. The balloon rises to a point in the atmosphere where the pressure is 325 torr and the temperature is -37°C. What is the change in volume of the balloon as it ascends from 1.40 atm to a pressure of 325 torr? Change in volume 1 pt 1 pt Submit Answer Try Another Version 9 item attempts remaining 1 ptarrow_forwardA sample of hydrogen gas occupies a volume of 9.67 L at 51.0°C and 0.630 atm. If it is desired to increase the volume of the gas sample to 10.8 L, while decreasing its pressure to 0.453 atm, the temperature of the gas sample at the new volume and pressure must be _______ °C. Submit Answerarrow_forward
- [References] A tank contains a mixture of 51.9 g oxygen gas and 46.1 g carbon dioxide gas at 28.0°C. The total pressure in the tank is 8.58 atm. Calculate the partial pressures of each gas in the container. P(O2) = atm P(CO2) = atm Submit Answer Try Another Version 3 item attempts remainingarrow_forwardme Submit Answer $ A sample of methane gas collected at a pressure of 302 mm Hg and a temperature of 290 K has a mass of 10.2 grams. The volume of the sample is L. 4 R % 5 T east.cengagenow.com [Review Topics] [References] Use the References to access important values if needed for this question. Retry Entire Group 9 more group attempts remaining Cengage Learning Cengage Technical Support ^ 6 MacBook Pro 18⁰ & 7 YU * OWLV2 | Online teaching and learning resource from Cengage Learning 8 9 ( O 0 4 P Previous Next> Email Instructor Save and Exit deletearrow_forward1 mix 2.6 grams of nitrogen, 4.73 grams of hydrogen and 5.33 grams of helium in a flask. If the total pressure in the system was found to be 205 kPa. How much pressure of the total does each gas exert? a) The pressure due to the hydrogen was Submit Answer Tries 0/99 b) The pressure due to the nitrogen was Submit Answer Tries 0/99 c) The pressure due to the helium was Submit Answer Tries 0/99 M CAL 8:00- This discussion is closed. 2. 10:50- esc CI 12:00 A ! 1 Q A F1 21 @ 2 S M F2 # 3 E D 80 F3 $ 4 R F a F4 % 5 T G F5 A 6 Y H tv MacBook Air C F6 & 7 U « F7 * 00 8 1 DII FB ( 9 - O DD F9 ) O A F10 P 4 F11 { [ + = F12 } 1 Send Feedback deletearrow_forward
- A sample of a gas mixture contains the following quantities of three gases. compound mass CO 2.33 CO2 3.40 g SF6 3.44 g The sample has: volume = 2.50 L temperature = 16.6 °C What is the partial pressure for each gas, in mmHg? What is the total pressure in the flask? CO mmHg CO2 mmHg SF6 mmHg total mmHg Submit Show Approach Show Tutor Steps Submit Answer Try Another Version 6 item attempts remainingarrow_forward212 n A sample of 11.8 liters of an ideal gas at 27.0 °C and 740.5 torr is compressed and heated so that the volume is 6.90 liters and the = tota temperature is 70.0 °C. 1st attempt 12 lil See Periodic Table O See Hi What is the pressure in the container? torrarrow_forwardhow do i solve this?arrow_forward
- pt A sample of hydrogen gas that occupies a volume of 12.5 L at a temperature of 0 °C and a pressure of I atm contains moles of gas. ot ot Submit Answer Try Another Version 2 item attempts remaining ot ot Untitled document (4) pdf Open file ANNOTATED BIBLI..docx Untitled document (3) pdf Open file ANNOTATED BIBLI..doox MG 5574jpg Open file ... Open file ... Open file search 立arrow_forwardA mixture of krypton and helium gases, in a 6.73 L flask at 88 °C, contains 9.60 grams of krypton and 0.660 grams of helium. The partial pressure of helium in the flask is atm and the total pressure in the flask is atm. Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardPlease correct answer and don't use hend raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY