
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
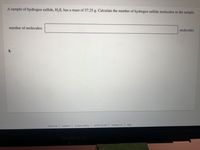
Transcribed Image Text:A sample of hydrogen sulfide, H2S, has a mass of 57.25 g. Calculate the number of hydrogen sulfide molecules in the sample.
number of molecules:
molecules
terms of use contact us
| help
about us
privacy policy
careers
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Crime scene investigators keep a wide variety of compounds on hand to help with identifying unknown substances they find in the course of their duties. One such investigator, while reorganizing their shelves, has mixed up several small vials and is unsure about the identity of a certain powder. Elemental analysis of the compound reveals that it is 67.31 % carbon, 6.978% hydrogen, 4.617% nitrogen, and 21.10% oxygen by mass. Which of the compounds could the powder be? C17H19NO3C17H19NO3 = morphine, analgesic C17H21NO4C17H21NO4 = cocaine, illicit drug C7H5N3O6C7H5N3O6 = 2,4,6-trinitrotoluene (TNT), commonly used explosive C10H15NC10H15N = methamphetamine, stimulant C4H5N2OC4H5N2O = caffeine, stimulant C11H15NO2C11H15NO2 = 3,4-methylenedioxymethamphetamine (MDMA), illicit drug C21H23NO5C21H23NO5 = heroin, illicit drug C3H6NO3C3H6NO3 = hexamethylene triperoxide diamine (HMTD), commonly used explosivearrow_forwardPart A. What is the mass of 9.50 moles of magnesium chloride, MgCl2? Express your answer with appropriate units. Part B. How many moles of nitrogen, N, are in 76.0g of nitrous oxide, N2O? Express your answer with the appropriate units.arrow_forwardChlorophyll molecules are responsible for the green pigmentation of plants. Differing chemically isolated molecular chlorophyll compounds are named as "Chlorophyll X" (where "X" is a variable denoting the actual molecular fragments which are represented by the labels R2, R3, R7, Rg, R17). Suppose one molecule of ChlorophyllX contains 5 oxygen atoms, and Chlorophyll X is 13.14 % oxygen by weight. Using only this information and the atomic mass of oxygen, determine the molecular mass of Chlorophyll X. The Chlorophyll X molecule contains one magnesium atom. What mass of magnesium is found in 7.11 g of Chlorophyll X compound?arrow_forward
- You wish to prepare 207 grams of 14.7 % Ca(NO3)2. You will need grams of calcium nitrate and mL of water.Assume that the density of water is 1.00 g / ml.arrow_forwardA 3.000 g sample of a gaseous compound was found to contain 2.560 g of carbon and 0.440 g of hydrogen. A) What is the simplest formula of the compound (Write C first and then H)? You won't be able to make the numbers a subscript. For instance, for MgCl2 enter MgCl2. B) The molar mass of the compound was found to be 42.08 g/mol. What is the molecular formula of the compound? You won't be able to make the numbers a subscript. For instance, for MgCl2 enter MgCl2.arrow_forwardCalculate the mass (in kilograms) of chlorine in 25 kg of chlorofluorocarbon (CFC). CFCl3arrow_forward
- A scientist is trying to discover information about an unknown metal in a compound. The formula for the compound is believed to be XBr, where X is the unknown metal. The scientist determined that a 4.723 g sample of this compound contains 5.313 x 10-2 mol Br. Calculate the atomic mass of the unknown metal, X. atomic mass= What is the identity of the metal? Provide the name or symbol of the element. metal: MacBook Pro amuarrow_forwardWhen 2.013 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 6.315 grams of CO2 and 2.586 grams of H2O were produced. In a separate experiment, the molar mass of the compound was found to be 70.13 g/mol. Determine the empirical formula and the molecular formula of the hydrocarbon. Enter the elements in the order presented in the question. empirical formula = molecular formula =arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY